1. Introduction
Zeolites are widely used in catalytic and separation processes due to their unique properties. They are used for their solid acid properties but also for their highly ordered micropore structures that enable shape and size selectivity [
1,
2]. Zeolites are made of three-dimensional crystalline networks containing channels and cages with sizes on the order of nanometers. Whereas these nanosized confinements provide the zeolite its unique properties, for certain applications, the transport of reactants and products through these channels can be the rate-limiting step. In order to improve access to the active sites, research on improving the zeolite pore network has been conducted during the last decades, an area referred to as hierarchical zeolites [
3,
4]. It generally consists of reducing the size of the microporous domains to shorten the mean diffusion path length. Many different approaches have been proposed to achieve this goal [
5,
6,
7,
8], but other important zeolite properties, such as the surface area, Brønsted acidity, Lewis acidity, and site density, are changed as well. It is therefore not always clear if the improved performance is specifically due to improved site accessibility, and this remains a challenging task [
9].
Therefore, the use of a well-defined model reaction that is impacted by the porosity and acid strength of the zeolite might aid in the rational development of mesoporous zeolites. Different model reactions have been used to characterize the acidity and/or porosity of zeolites [
9,
10]. Weisz et al. [
11,
12,
13] suggested the cracking of
n-hexane at certain conditions (temperature, contact time) to assess the acidity and rank solid acid catalysts according to their relative activity. This α-test can measure the rate constant for
n-hexane cracking over four orders of magnitude. However, it does not differentiate between the number of acid sites and the acid strength, nor does the test provide information on possible pore diffusion limitations.
Several studies report the use of
n-hexane cracking to characterize the performance of mesoporous HZSM-5 catalysts, analyzing the data in terms of turnover frequency, coke formation rate and/or diffusion limitations [
14,
15,
16,
17]. Only a few studies for zeolite catalyzed reactions use models that are quantitatively based on zeolite properties such as acid strength and porosity [
18,
19,
20,
21,
22,
23,
24,
25]. Yaluris et al. developed a detailed microkinetic model for the cracking of 2-methyl-hexane over 6 USY FCC catalysts with increasing steaming severity. The activity and selectivity could be correctly described over the six catalysts with the acid strength as a key parameter [
18]. Thybaut et al. described alkane hydroconversion over Pt/H–(US)Y zeolites with a kinetic model that included alkene protonation enthalpy to account for varying acid strength [
19]. Borges et al. studied
n-hexane cracking over a series of HZSM-5 catalysts with different acid strength distributions. They quantified their data by a Polanyi linear energy relationship [
20]. Iglesia and colleagues developed a theoretical framework linking the activation energy for acid-catalyzed reactions to the deprotonation energy (DPE), which is a descriptor of the Brønsted acid strength, and applied this to several reactions [
21,
22,
23]. More recently, they extended the model by including the Thiele modulus to account for transport by diffusion [
24,
25].
This paper focuses on developing a detailed but simple kinetic analysis based on three catalyst descriptors: the number of Brønsted acid sites, the porosity, and the acid strength in order to predict the conversion of n-hexane cracking over different mesoporous HY zeolites. The rate equation is based on the fundamental reaction mechanism for protolytic alkane cracking over porous solid acid catalysts, accounting for physisorption, diffusion, protonation, and cracking. Several mesoporous HY zeolites were synthesized and characterized by well-established protocols for this purpose.
3. Discussion
n-Hexane cracking can proceed via a monomolecular or bimolecular reaction mechanism. Babitz et al. [
35] showed that the
n-hexane conversion was first-order over H-USY, following monomolecular cracking.
Figure S10 shows the product selectivity as a function of the conversion for all four samples. The product distribution as a function of the conversion is very similar for all four samples and is similar to those reported by Babitz et al. [
35]. This shows that
n-hexane cracking proceeds by monomolecular cracking. The protonation of
n-hexane leads to the formation of an alkylcarbonium ion that is transformed by protolytic cracking into a smaller alkylcarbenium ion and a smaller alkane or hydrogen. The alkylcarbenium ion can deprotonate into an alkene or undergo consecutive reactions (isomerization, hydride transfer, or cracking).
In the case of monomolecular cracking, a first comparison of the catalytic activities of the four samples can be performed based on the α-test. This consists of calculating the volumetric rate constants (s
−1) for
n-hexane cracking at 811 K (1000 °F, which is the temperature defined for the α-test [
11]), for contact times that lead to conversion levels between 5% and 20% and normalizing them with respect to the value of the rate constant for
n-hexane cracking over amorphous silica-alumina [
11].
The volumetric rate constant at 811 K is calculated from the integrated first-order rate equation as:
where: X
n−C6 is the
n-hexane conversion at 811 K, and τ
s the superficial contact time (s), calculated as the ratio of the catalyst volume and the volumetric flow rate of
n-hexane.
To calculate the relative cracking activity α of the H-Y and H-USY zeolites, a value of 0.012 s
−1 for the rate constant for
n-hexane cracking over an amorphous silica-alumina was estimated, according to the reaction conditions reported in [
11]. The conversion data were extrapolated to 811 K with the corresponding activation energies.
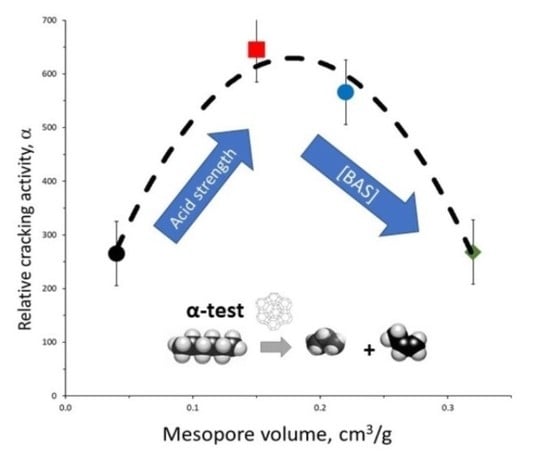
Figure 6 shows the relative cracking activity as a function of the mesopore volume. A volcano-like curve is observed. An optimum activity appears around a mesoporosity of 0.15, corresponding to the H-USY-0 sample. This is likely due to a trade-off between the increasing acidity and/or rate of molecular transport in the mesoporous materials and the decrease in the number of Brønsted sites with increasing mesoporosity.
Protolytic cracking only occurs over Brønsted acid sites, as the first step consists of the transfer of a proton to form the penta-coordinated carbenium ion. Linear correlations between the cracking activity and the number of framework aluminum sites have been observed for conventional and dealuminated faujasite zeolites [
36]. LAS does not seem to have a significant impact on the activity in our case either, as the catalyst with the highest activity contains the lowest number of LAS (H-USY-0,
Table 4). Thus, the activity per acid site can be calculated from the volumetric rate constant corrected for the number of Brønsted acid sites, N
BAS (mol/m
3), determined by pyridine FT-IR and reported in
Table 5.
Note that k
V is not an intrinsic cracking rate constant but is lumped with the
n-hexane physisorption equilibrium constant. De Moor et al. provide a theoretical value of the
n-hexane physisorption equilibrium constant at 500 °C of 0.9 MPa
−1 for faujasite, which agreed well with various experimental values [
37]. Using a Langmuir isotherm, coverages of adsorbed
n-hexane of less than 0.01 for all samples ≥ 500 °C can be estimated (Henry regime). As very similar values of the physisorption enthalpy were measured for all samples (
Table 4), the lumped rate coefficient, k
V, and associated with the apparent activation energy are used for the kinetic analysis.
The values of k
V are shown as an Arrhenius plot in
Figure 7. The data obey Arrhenius law, indicating that no significant change in mechanism occurs in this temperature range. The values of the rate constants for H-USY-0, H-USY-1, H-USY-2 are very similar, as well as the values of the apparent activation energy (129.7 ± 2 kJ/mol). Note that due to the lumping with the physisorption constant, the values of intrinsic activation energies will be approximately 40 kJ/mol higher. The values of the apparent activation energies range between 129 and 132 kJ/mol, in suitable agreement with the values of 123–128 kJ/mol reported by Konno et al. [
15] for H-ZSM-5, but lower than the value of 177 kJ/mol reported by Babitz et al. [
35] for
n-hexane cracking over H-USY. The values of the rate constant at 923 K are also close to the values reported by Konno et al. (6 × 10
−3 vs. 1 × 10
−2 m
3/kg/s) [
15].
The similar values of the rate constants normalized per Brønsted acid site and activation energies for the H-USY zeolites indicate that the drop in relative activity for
n-hexane cracking per catalyst volume with increasing mesopore volume from 0.15 to 0.32 cm
3/g,
Figure 6, is due to the decreasing concentration of Brønsted acid sites (
Table 4).
The value of the rate constant for the microporous H-Y sample, on the other hand, is approximately two times smaller, and the activation energy is significantly lower (123.7 vs. 129.7 ± 2 kJ/mol) than for the H-USY samples. There can be several reasons for the lower activity of H-Y compared to the H-USY samples.
The lower activity of the H-Y sample could be due to internal diffusion limitations, as the H-Y has much larger microporous domains than the H-USY samples and, therefore, a longer diffusion time ((L
D)
2/D). Assuming that no diffusion limitation occurs over H-USY-1 due to the small microporous domains, then the ratio of the rate constants, k
V, between H-Y and H-USY-1 corresponds to the effectiveness factor of the H-Y sample. This yields a value of the effectiveness factor of 0.5 at 550 °C, which, for spherical H-Y particles, corresponds to a Thiele modulus of 4.6 [
38]. By repeating this calculation at different temperatures, the activation energy for the internal diffusion coefficient of approximately 120 kJ/mol can be estimated. This value is much higher than the values of the
n-hexane physisorption enthalpy of ~−40 kJ/mol on H-Y and H-USY (
Table 4). Activation energies for diffusion (E
a,dif) are usually smaller than the value for the physisorption enthalpy, as the physisorption enthalpy corresponds to the energy required to completely remove the molecule from the zeolite force field. In the diffusion-limited regime, the observed activation energy is given by [
38]:
This equation yields, with a more realistic value of the activation energy for diffusion (~20 kJ/mol), a much lower value of the apparent activation energy (~75 kJ/mol) for n-hexane cracking than measured experimentally. Thus, the lower activity is not (only) due to internal diffusion limitations.
The lower strength of acid sites might be responsible for the lower value of the rate constant.
Figure SI-9 shows that pyridine desorption on the Brønsted sites as a function of temperature was faster over the H-Y sample than over the H-USY samples. This implies a lower adsorption enthalpy for pyridine adsorption on Brønsted sites for the H-Y sample than for the H-USY samples, indicating a lower acid strength. The effect of the acid strength on the cracking rate of small paraffin molecules has been studied extensively in the literature [
18,
19,
20,
21,
22,
23,
24,
25,
39,
40,
41], with contradicting viewpoints. Some studies show that there is no effect of the acid strength on the cracking rate [
16,
17]. In fact, several experimental and modeling studies show similar activation energies for very different zeolites, ruling out any effect of acid strength on the cracking rate.
Theoretical calculations show that the acid strength, expressed as the deprotonation energy (DPE), for Y zeolites (1161–1166 kJ mol
−1) is independent of the Al framework location and increases only in the case of next-nearest-neighbor Al locations (1177–1247 kJ mol
−1) [
42]. It is admitted that Y zeolites with Si/Al > 5 are mainly constituted of isolated Al ions [
40]. Note that the Si/Al of the H-Y sample is 2.5, while the H-USY samples are around 15. The H-Y zeolite likely contains next-nearest-neighbor Al ions with lower acid strength. However, if the low activity of the H-Y sample is fully attributed to the acid strength, a value of the activation energy higher than that of the H-USY samples would be expected on the basis of the results in [
18,
19,
20,
21,
22,
23,
24,
25], while the opposite has been observed here (
Figure 8, 123.7 < 129.7 ± 2 kJ/mol).
Several studies show that mild steaming of cracking catalysts lead to an increase in its activity [
13,
36,
41]. Steaming removes Al ions from the framework, accompanied by a stability increase and the formation of extra-framework aluminum species (EFAL) [
40]. Different explanations have been provided on the enhanced activity; the increase in the activity has been correlated with the number of paired Al sites or by an inductive influence of the Lewis acidity created by EFAL species on the hydroxyl groups [
40]. Even when the acid strength is lowered by interaction with EFAL species, an increase in the activation energy would be expected.
The relation between the apparent activation energy and the deprotonation energy (DPE), which is a descriptor of the Brønsted acid strength, can be visualized by constructing a Born-Haber thermochemical cycle for
n-hexane cracking as an energy diagram, as shown in
Figure 8 [
19,
21].
Figure 8 shows the enthalpy levels of the different reactants and intermediates for alkane cracking over Brønsted acid sites. The reaction path consists of the physisorption of the alkane, R(g), from the gas phase to the physisorbed state, R(z), followed by the protonation of R(z) to an alkylcarbonium ion, R-H
+(z), shown as a transition state. The alkylcarbonium ion will then crack into a smaller alkylcarbenium ion and a smaller alkane or hydrogen. The diagram shows that the intrinsic barrier (E
a) is equal to the sum of the physisorption enthalpy (ΔH
phys) and the apparent activation energy (E
a,app).
A different independent path can be chosen to reach the same transition state since the diagram concerns thermodynamical quantities. This path involves the DPE, the proton affinity (ΔH
PA), and the stabilization energy (E
stab) [
19,
21,
22,
23]. It follows from the diagram in
Figure 8 that the sum of the apparent activation energy (E
a,app), the stabilization energy, and the proton affinity is equal to the DPE. Thus, an increase in the DPE, which corresponds to a weaker acid strength (DPE
weak), and assuming constant stabilization energy, will lead to an increase in the apparent activation energy (as well as the intrinsic barrier). This is indicated by the red dotted lines in
Figure 8.
Generally, the stabilization energy will also change with changing acid strength, resulting in a less than one to one dependence of the apparent activation energy on the DPE [
21]. The sum of the DPE and the stabilization energy is sometimes taken as an indicator of the acid strength [
18].
As explained above, a lower acid strength will lead to an increase, while diffusion limitations will lead to a decrease in the activation energy. Thus, the small change in the value of the activation energy for
n-hexane cracking over H-Y compared to the H-USY samples might be due to both a lower acid strength and internal diffusion limitations. To further verify this assumption, the H-Y conversion data at different temperatures were fitted to the integrated first-order equation, including the effectiveness factor:
k
V is given as a function of the temperature by:
Both E
a,app and (L
D)
2/D can be estimated by regression analysis of Equations (5–10) with the experimental conversion data for the HY zeolite. The value of N
BAS (376 mol/m
3) of the H-Y zeolite was used. For the pre-exponential factor, k
0V, the value for the H-USY-1 zeolite (7.3 10
6 s
−1) was used, as this data was considered as the intrinsic rate. Macht et al. [
21] reported that the acid strength does affect the activation energy, but not the pre-exponential factor. Furthermore, it was assumed that the value of the diffusion coefficient, D is constant over the temperature interval (E
a,dif = 0), as it was found that the value of E
a,dif did not impact the estimated E
a,app value. The estimated values of E
a,app, and (L
D)
2/D are listed in
Table 6. The value for E
a,app (138.7 kJ/mol) is now indeed higher than that of H-USY-1 due to the lower acid strength of the H-Y sample.
Macht et al. [
21] found for
n-hexane isomerization over Keggin-type tungsten polyoxometalates and H-BEA zeolite the following relation between the change in DPE and the apparent activation energy:
Assuming that this relation also holds for
n-hexane cracking over Y zeolites and taking the H-USY-1 zeolite as the reference, the following relation for the apparent activation energy can be derived:
where ∆DPE corresponds to the difference in protonation energy between the HY and H-USY-1 samples. This equation gives a value of ∆DPE of 23 kJ/mol. The value of 23 kJ/mol for ∆DPE is well within the range of 86 kJ/mol of the DPE variation reported for the next-nearest-neighbor Al atoms [
42] in Y zeolites. Yaluris et al. [
18] found a variation in the protonation energy of 26 kJ/mol for FCC catalysts that underwent different severities of steaming, while Thybaut et al. found a change of 20 kJ/mol for the ∆DPE between microporous HY and mesoporous H-USY for n-octane hydroisomerization [
19].
The effectiveness factors over the H-Y sample at 550, 575, 600, and 650 °C were 0.98, 0.96, 0.95, and 0.84, respectively, indicating that some diffusion limitation (η < 0.95) occurs only at the highest temperature of 650 °C.
Thus, the introduction of the mesoporosity from H-Y to H-USY-0 leads to a higher cracking rate mainly due to the change in acid strength. The change in acid strength can be due either to site isolation or the effect of the increasing EFAL concentration on the Brønsted sites.