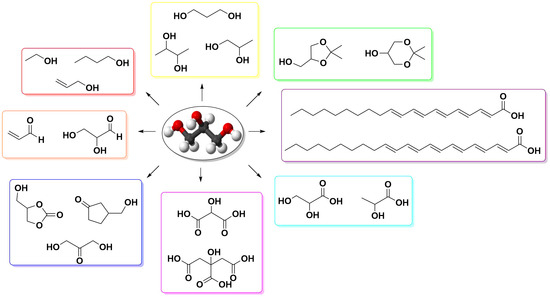
A Focus on the Transformation Processes for the Valorization of Glycerol Derived from the Production Cycle of Biofuels
Abstract
:1. Introduction
2. Conversion of Glycerol under Batch Conditions
3. Biological Conversion of Glycerol Supported by Microorganisms
4. Conversion of Glycerol Supported by Catalysts
5. Products Arising from Glycerol by Continuous Flow Procedures
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Van Boi, L.; Maeda, Y. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalyst 2012, 2, 191–222. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, C.; Andresini, M.; DeGennaro, L.; Luisi, R. Benchmarking Acidic and Basic Catalysis for a Robust Production of Biofuel from Waste Cooking Oil. Catalyst 2019, 9, 1050. [Google Scholar] [CrossRef] [Green Version]
- Kar, R.; Gupta, O.; Das, M.K. Biodiesel production and process optimization. IJSRP Int. J. Sci. Res. Publ. 2012, 2, 2250–3153. [Google Scholar]
- Enweremadu, C.; Mbarawa, M. Technical aspects of production and analysis of biodiesel from used cooking oil—A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Tomasevic, A.; Siler-Marinkovic, S. Methanolysis of used frying oil. Fuel Process. Technol. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef] [Green Version]
- Hsu, A.-F.; Jones, K.; Marmer, W.N.; Foglia, T.A. Production of alkyl esters from tallow and grease using lipase immobilized in a phyllosilicate sol-gel. J. Am. Oil Chem. Soc. 2001, 78, 585–588. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol: New Usages for a Versatile Raw Material, 2nd ed.; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Ahmed, S.; Papalias, D. Hydrogen from Glycerol: A Feasibility Study Presented at the 2010 Hydrogen Program Annual Merit Review Meeting Washington, D.C. U.S. Patent No. PD003, 8 June 2010. [Google Scholar]
- Leoneti, A.B.; Aragão-Leoneti, V.; de Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energy 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Bottari, G.; Barta, K. Lactic acid and hydrogen from glycerol via acceptorless dehydrogenation using homogeneous catalysts. Recycl. Catal. 2015, 2, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Aroua, M.; Daud, W.; Cognet, P.; Pérès-Lucchese, Y.; Fabre, P.-L.; Reynes, O.; Latapie, L. A review: Conversion of bioglycerol into 1,3-propanediol via biological and chemical method. Renew. Sustain. Energy Rev. 2015, 42, 963–972. [Google Scholar] [CrossRef] [Green Version]
- García, J.I.; Fraile, J.M.; Herrerías, C.I.; Pires, E. Synthetic Transformations for the Valorization of Fatty Acid Derivatives. Synthesis 2017, 49, 1444–1460. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Aziz, A.A.; Aroua, M. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Yang, F.; A Hanna, M.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Jérôme, F.; Pouilloux, Y.; Barrault, J. Rational Design of Solid Catalysts for the Selective Use of Glycerol as a Natural Organic Building Block. ChemSusChem 2008, 1, 586–613. [Google Scholar] [CrossRef]
- Chalmers, W.; Wood, A.C.; Shaw, A.J.; Majnarich, J.J. Treatment of Inflammatory Diseases. U.S. Patent No. US3294639A, 27 December 1966. [Google Scholar]
- Haynes, M.P.; Buckley, H.R.; Higgins, M.L.; Pieringer, R.A. Synergism between the antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob. Agents Chemother. 1994, 38, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Boeryd, B.; Hallgren, B.; Ställberg, G. Studies on the effect of methoxy-substituted glycerol ethers on tumor growth and metastasis formation. Br. J. Exp. Pathol. 1971, 52, 221–230. [Google Scholar]
- Weber, N. Progress in Biochemical Pharmacology; Braquet, P., Mangold, H.K., Vargaftig, B.B., Eds.; Karger: Basel, Switzerland, 1988; Volume 22, pp. 48–57. [Google Scholar]
- Brohult, A.; Brohult, J.; Brohult, S.; Joelsson, I. Reduced Mortality in Cancer Patients after Administration of Alkoxyglycerols. Acta Obstet. Gynecol. Scand. 1986, 65, 779–785. [Google Scholar] [CrossRef]
- Costa, I.C.R.; Itabaiana, I.; Flores, M.C.; Lourenço, A.C.; Leite, S.G.F.; Miranda, L.S.D.M.E.; Leal, I.C.R.; De Souza, R.O.M.A. Biocatalyzed Acetins Production under Continuous-Flow Conditions: Valorization of Glycerol Derived from Biodiesel Industry. J. Flow Chem. 2013, 3, 41–45. [Google Scholar] [CrossRef]
- Pradima, J.; Kulkarni, M.R.; Archna. Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resour. Technol. 2017, 3, 394–405. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Papanikolaou, S. Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol. 2012, 95, 13–27. [Google Scholar] [CrossRef]
- Bell, B.M.; Briggs, J.R.; Campbell, R.M.; Chambers, S.M.; Gaarenstroom, P.D.; Hippler, J.G.; Hook, B.D.; Kearns, K.; Kenney, J.M.; Kruper, W.J.; et al. Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process. CLEAN Soil Air Water 2008, 36, 657–661. [Google Scholar] [CrossRef]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Wilkens, E.; Ringel, A.K.; Hortig, D.; Willke, T.; Vorlop, K.-D. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063. [Google Scholar] [CrossRef]
- Papanikolaou, S. High production of 1,3-propanediol from industrial glycerol by a newly isolated Clostridium butyricum strain. J. Biotechnol. 2000, 77, 191–208. [Google Scholar] [CrossRef]
- Menzel, K.; Zeng, A.-P.; Deckwer, W.-D. High concentration and productivity of 1,3-propanediol from continuous fermentation of glycerol by Klebsiella pneumoniae. Enzym. Microb. Technol. 1997, 20, 82–86. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Sang, B.-I.; Um, Y. Butanol production from thin stillage using Clostridium pasteurianum. Bioresour. Technol. 2011, 102, 4934–4937. [Google Scholar] [CrossRef]
- Kao, W.-C.; Lin, D.-S.; Cheng, C.-L.; Chen, B.-Y.; Lin, C.-Y.; Chang, J.-S. Enhancing butanol production with Clostridium pasteurianum CH4 using sequential glucose–glycerol addition and simultaneous dual-substrate cultivation strategies. Bioresour. Technol. 2013, 135, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, R.; Alves, M.; Rodrigues, L. Modulation of crude glycerol fermentation by Clostridium pasteurianum DSM 525 towards the production of butanol. Biomass Bioenergy 2014, 71, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Petrov, K.; Petrova, P. Enhanced production of 2,3-butanediol from glycerol by forced pH fluctuations. Appl. Microbiol. Biotechnol. 2010, 87, 943–949. [Google Scholar] [CrossRef]
- Yang, T.-W.; Rao, Z.-M.; Zhang, X.; Xu, M.-J.; Xu, Z.-H.; Yang, S.-T. Fermentation of biodiesel-derived glycerol by Bacillus amyloliquefaciens: Effects of co-substrates on 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2013, 97, 7651–7658. [Google Scholar] [CrossRef]
- Cho, S.; Kim, T.; Woo, H.M.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Ding, Y.; Xian, M.; Xu, X.; Zhang, R.; Zhao, G. Production of optically pure d -lactate from glycerol by engineered Klebsiella pneumoniae strain. Bioresour. Technol. 2014, 172, 269–275. [Google Scholar] [CrossRef]
- Murakami, N.; Oba, M.; Iwamoto, M.; Tashiro, Y.; Noguchi, T.; Bonkohara, K.; Abdel-Rahman, M.A.; Zendo, T.; Shimoda, M.; Sakai, K.; et al. l-Lactic acid production from glycerol coupled with acetic acid metabolism by Enterococcus faecalis without carbon loss. J. Biosci. Bioeng. 2016, 121, 89–95. [Google Scholar] [CrossRef]
- Mazumdar, S.; Blankschien, M.D.; Clomburg, J.M.; Gonzalez, R. Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb. Cell Factories 2013, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Pyle, D.J.; Garcia, R.A.; Wen, Z. Producing Docosahexaenoic Acid (DHA)-Rich Algae from Biodiesel-Derived Crude Glycerol: Effects of Impurities on DHA Production and Algal Biomass Composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [Google Scholar] [CrossRef]
- Abad, S.; Turon, X. Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: Focus on polyunsaturated fatty acids. Biotechnol. Adv. 2012, 30, 733–741. [Google Scholar] [CrossRef]
- Athalye, S.K.; Garcia, R.A.; Wen, Z. Use of Biodiesel-Derived Crude Glycerol for Producing Eicosapentaenoic Acid (EPA) by the Fungus Pythium irregulare. J. Agric. Food Chem. 2009, 57, 2739–2744. [Google Scholar] [CrossRef]
- Sarris, D.; Philippoussis, A.; Mallouchos, A.; Diamantopoulou, P. Valorization of low-cost, carbon-rich substrates by edible ascomycetes and basidiomycetes grown on liquid cultures. FEMS Microbiol. Lett. 2020, 367, 168. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.-P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973–1983. [Google Scholar] [CrossRef]
- O’Grady, J.; Morgan, J.A. Heterotrophic growth and lipid production of Chlorella protothecoides on glycerol. Bioprocess Biosyst. Eng. 2010, 34, 121–125. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour. Technol. 2002, 82, 43–49. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, 063. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.; de Almeida, M.C.M.; Grandfils, C.; da Fonseca, M. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process. Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Ibrahim, M.H.A.; Steinbüchel, A. Poly(3-Hydroxybutyrate) Production from Glycerol by Zobellella denitrificans MW1 via High-Cell-Density Fed-Batch Fermentation and Simplified Solvent Extraction. Appl. Environ. Microbiol. 2009, 75, 6222–6231. [Google Scholar] [CrossRef] [Green Version]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, A.R. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Jitrwung, R.; Yargeau, V. Biohydrogen and Bioethanol Production from Biodiesel-Based Glycerol by Enterobacter aerogenes in a Continuous Stir Tank Reactor. Int. J. Mol. Sci. 2015, 16, 10650–10664. [Google Scholar] [CrossRef] [Green Version]
- Sarris, D.; Papanikolaou, S. Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.J.; Hartono, M.R.; Chan, W.H.; Yeo, S.S. Ethanol production from biodiesel-derived crude glycerol by newly isolated Kluyvera cryocrescens. Appl. Microbiol. Biotechnol. 2011, 89, 1255–1264. [Google Scholar] [CrossRef]
- Oh, B.-R.; Seo, J.-W.; Heo, S.-Y.; Hong, W.-K.; Luo, L.H.; Joe, M.-H.; Park, D.-H.; Kim, C.H. Efficient production of ethanol from crude glycerol by a Klebsiella pneumoniae mutant strain. Bioresour. Technol. 2011, 102, 3918–3922. [Google Scholar] [CrossRef]
- Metsoviti, M.; Paraskevaidi, K.; A Koutinas, A.; Zeng, A.-P.; Papanikolaou, S. Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Process. Biochem. 2012, 47, 1872–1882. [Google Scholar] [CrossRef]
- Metsoviti, M.; Paramithiotis, S.; Drosinos, E.H.; Galiotou-Panayotou, M.; Nychas, G.-J.E.; Zeng, A.-P.; Papanikolaou, S. Screening of bacterial strains capable of converting biodiesel-derived raw glycerol into 1,3-propanediol, 2,3-butanediol and ethanol. Eng. Life Sci. 2011, 12, 57–68. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Dabdawb WA, Y.; Mansouri, N. Biodiesel-Derived Raw Glycerol to Value-Added Products: Catalytic Conversion Approach. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Wiley: Hoboken, NJ, USA, 2017; Volume 3, pp. 309–366. [Google Scholar]
- Goetsch, D.; Machay, I.S.; White, L.R. Production of Methanol from the Crude Glycerol by-Product of Producing Biodiesel. U.S. Patent No. US7388034B1, 17 June 2008. [Google Scholar]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerin by steam reforming over nickel catalysts. Renew. Energy 2008, 33, 1097–1100. [Google Scholar] [CrossRef]
- Jagadeeswaraiah, K.; Chowdari, R.K.; Rajashekar, A.; Srivani, A.; Lingaiah, N. The Role of Tungsten Oxide Species Supported on Titania Catalysts for the Synthesis of Glycerol Carbonate from Glycerol and Urea. Catal. Lett. 2016, 146, 692–700. [Google Scholar] [CrossRef]
- Su, X.; Lin, W.; Cheng, H.; Zhang, C.; Wang, Y.; Yu, X.; Wu, Z.; Zhao, F. Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate. Green Chem. 2017, 19, 1775–1781. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Mohamed, M.; Ting, T.S.; Amin, N.A.S.; Abdullah, T.A.T.; Mat, R. Conversion of glycerol to methanol in the presence of zeolite based catalysts. In Proceedings of the 2011 IEEE Conference on Clean Energy and Technology (CET), Kuala Lumpur, Malaysia, 27–29 June 2011; pp. 389–393. [Google Scholar] [CrossRef]
- Finn, M.; Ridenour, J.A.; Heltzel, J.; Cahill, C.; Voutchkova-Kostal, A. Next-Generation Water-Soluble Homogeneous Catalysts for Conversion of Glycerol to Lactic Acid. Organometallics 2018, 37, 1400–1409. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Devi, B.P.; Prasad, R.; Prasad, P.S.; Lingaiah, N. Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol. Appl. Catal. A Gen. 2010, 384, 107–114. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Miedziak, P.J.; Douthwaite, M.; Brett, G.L.; Davies, T.E.; Morgan, D.J.; Edwards, J.K.; Knight, D.W.; Kiely, C.J.; Taylor, S.H.; et al. Base-Free Oxidation of Glycerol Using Titania-Supported Trimetallic Au-Pd-Pt Nanoparticles. ChemSusChem 2013, 7, 1326–1334. [Google Scholar] [CrossRef]
- Oger, N.; Lin, Y.F.; Rataboul, F.; Le Grognec, E.; Felpin, F.-X. Graphene-promoted acetalisation of glycerol under acid-free conditions. Green Chem. 2015, 18, 1531–1537. [Google Scholar] [CrossRef]
- Tao, M.; Sun, N.; Li, Y.; Tong, T.; Wielicako, M.; Wang, S.; Wang, X. Heteropolyacids embedded in a lipid bilayer covalently bonded to graphene oxide for the facile one-pot conversion of glycerol to lactic acid. J. Mater. Chem. A 2017, 5, 8325–8333. [Google Scholar] [CrossRef]
- Haider, M.H.; Dummer, N.F.; Knight, D.W.; Jenkins, R.L.; Howard, M.; Moulijn, J.; Taylor, S.H.; Hutchings, G.J. Efficient green methanol synthesis from glycerol. Nat. Chem. 2015, 7, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Dang, L.; Lohrman, J.; Subramaniam, B.; Ren, S.; Chaudhari, R.V. Lattice-Matched Bimetallic CuPd-Graphene Nanocatalysts for Facile Conversion of Biomass-Derived Polyols to Chemicals. ACS Nano 2013, 7, 1309–1316. [Google Scholar] [CrossRef]
- Carlucci, C.; DeGennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalyst 2019, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Colella, M.; Carlucci, C.; Luisi, R. Supported Catalysts for Continuous Flow Synthesis. Top. Curr. Chem. 2018, 376, 46. [Google Scholar] [CrossRef]
- Martin, M.; Grassmann, I.E. ASI: Towards the optimal integrated production of biodiesel with internal recycling of methanol produced from glycerol. Environ. Prog. Sustain. Energy 2013, 32, 891–901. [Google Scholar] [CrossRef]
- Tshibalonza, N.N.; Monbaliu, J.C.M. Revisiting the deoxydehydration of glycerol towards allyl alcohol under continuous-flow conditions. Green Chem. 2017, 19, 3006–3013. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S. Biodiesel manufactoring. Chem. Process Des. Comput. Aided Case Stud. 2008, 14, 399–428. [Google Scholar]
- Bhanuchander, P.; Priya, S.S.; Kumar, V.P.; Hussain, S.; Rajan, N.P.; Bhargava, S.K.; Chary, K.V.R. Direct Hydrogenolysis of Glycerol to Biopropanols over Metal Phosphate Supported Platinum Catalysts. Catal. Lett. 2017, 147, 845–855. [Google Scholar] [CrossRef]
- Liebig, C.; Paul, S.; Katryniok, B.; Guillon, C.; Couturier, J.-L.; Dubois, J.-L.; Dumeignil, F.; Hoelderich, W.F. Glycerol conversion to acrylonitrile by consecutive dehydration over WO3/TiO2 and ammoxidation over Sb-(Fe,V)-O. Appl. Catal. B Environ. 2013, 132, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Len, C.; Delbecq, F.; Corpas, C.C.; Ramos, E.R. Continuous Flow Conversion of Glycerol into Chemicals: An Overview. Synthesis 2017, 50, 723–741. [Google Scholar] [CrossRef]
- Bühler, W.; Dinjus, E.; Ederer, H.; Kruse, A.; Mas, C. Ionic reactions and pyrolysis of glycerol as competing reaction pathways in near- and supercritical water. J. Supercrit. Fluids 2002, 22, 37–53. [Google Scholar] [CrossRef]
- Yuksel, A.; Koga, H.; Sasaki, M.; Goto, M. Hydrothermal Electrolysis of Glycerol Using a Continuous Flow Reactor. Ind. Eng. Chem. Res. 2010, 49, 1520–1525. [Google Scholar] [CrossRef]
- Nogueira, D.O.; De Souza, S.P.; Leão, R.A.C.; Miranda, L.S.M.; De Souza, R.O.M.A. Process intensification for tertiary amine catalyzed glycerol carbonate production: Translating microwave irradiation to a continuous-flow process. RSC Adv. 2015, 5, 20945–20950. [Google Scholar] [CrossRef]
- Mimura, N.; Muramatsu, N.; Hiyoshi, N.; Sato, O.; Masuda, Y.; Yamaguchi, A. Continuous Catalytic Oxidation of Glycerol to Carboxylic Acids Using Nanosized Gold/Alumina Catalysts and a Liquid-Phase Flow Reactor. ACS Omega 2018, 3, 13862–13868. [Google Scholar] [CrossRef] [PubMed]
- Guidi, S.; Noè, M.; Riello, P.; Perosa, A.; Selva, M. Towards a Rational Design of a Continuous-Flow Method for the Acetalization of Crude Glycerol: Scope and Limitations of Commercial Amberlyst 36 and AlF3·3H2O as Model Catalysts. Molecules 2016, 21, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
























| Acyl Donor a | Reaction Time | Conversion (%) a | Selectivity (%) a | ||
|---|---|---|---|---|---|
| (h) | (Yield %) | Mono | Di | Tri | |
| Acetic acid | 1 | 6 (2) | 6 | 0 | 0 |
| Vinyl acetate | 1 | 100 (92) | 0 | 98 | 2 |
| Ethyl acetate | 1 | 44 (38) | 42 | 2 | 0 |
| Acetic anhydride | 1 | 67 (58) | 50 | 17 | 0 |
| Acetic acid | 24 | 56 (50) | 46 | 10 | 0 |
| Vinyl acetate | 24 | 100 (92) | 0 | 81 | 19 |
| Ethyl acetate | 24 | 67 (60) | 51 | 16 | 0 |
| Acetic anhydride | 24 | 100 (94) | 0 | 54 | 46 |
| Compound | Microorganism | Concentration (g/L) | Yield (g/g) | Productivity (g/L/h) |
|---|---|---|---|---|
| 1,3-Propanediol | Clostridium butyricum Klebsiella pneumoniae | 35–93.7 | 0.51–0.55 | 2.3–8.8 |
| n-Butanol | Clostridium pasteurianum | 6–12 | 0.19–0.44 | 0.03–0.15 |
| 2,3-Butanediol | Klebsiella pneumoniae Klebsiella oxytoca Bacillus amyloliquefaciens | 43–132 | 0.39–0.44 | 0.45–0.84 |
| Lactic acid | Klebsiella pneumoniae Enterococcus faecalis Escherichia coli | 50–142.1 | 0.82–0.93 | 0.77–2.96 |
| DHA | Aurantiochytrium limacinum Schizocytrium limacinum | 2–5 | - | 0.026-0.038 |
| EPA | Pythium irregular | 0.09 | - | 6.21 × 10−4 |
| Mortierella ramanniana Mortierella isabelline | 4–5.4 | 0.16–0.22 | - | |
| Lipid | Cryptococcus curvatus Chlorella protohecoides | 23–24.6 | 0.31 | 0.06–0.13 |
| Yarrowia lipolytica Rhodosporidium toruloides | 3.5–12.5 | 0.16 | 0.12 | |
| PHB | Zobellella denitrificans Cupriavidus necator | 38.1–54.3 | 0.25 | 1.09–1.10 |
| Source | Microorganism | Concentration (g/L) | Yield (g/g) | Productivity (g/L/h) |
|---|---|---|---|---|
| Waste glycerol | Y. lipolytica A-101-1.22 | 112 | 0.6 | 0.71 |
| Waste glycerol | Y. lipolytica A-101-1.22 RB variant | 124.2 | 0.77 | 0.85 |
| Glycerol | Y. lipolytica NG40/UV5 | 87 | 0.64 | 0.906 |
| Waste glycerol | Y. lipolytica NG40/UV5 | 100 | 0.9 | 1.04 |
| Glycerol | Y. lipolytica LMBF Y-46 | 101.3 | 0.46 | - |
| Waste glycerol | Y. lipolytica LFMB 20 | 42 | 0.39 | - |
| Waste glycerol | Y. lipolytica ACA-DC 50109 | 62.5 | 0.56 | - |
| Glycerol | Y. lipolytica AWG7 | 154 | 0.74 | 1.05 |
| Yields (mole/mole GL) | Initial Glycerol | Reactor Type | SCAD/L of | |
|---|---|---|---|---|
| H2 | Ethanol | Concentration (g/L) | Media | |
| 0.96 | 0.90 | 15 | Batch–3.6 L | 0.91 |
| 0.86 | 0.75 | 15 | CSTR | 0.91 |
| Ni/MgO | Ni/CeO2 | Ni/Ti2O | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Glycerin Conversion | Conversion to Gases | Glycerin Conversion | Conversion to Gases | Glycerin Conversion | Conversion to Gases |
| 650 | 100 | 35 | 64 | 18 | 2 | 16 |
| 600 | 100 | 46 | 63 | 19 | 2 | 16 |
| 550 | 100 | 44 | 58 | 17 | 4 | 21 |
| Catalyst | Conversion | Selectivity (%) | |||
|---|---|---|---|---|---|
| (%) | 1,2-PD | EG | Acetol | Others | |
| 1 Ru/TiO2 (DP) | 35 | 64 | 18 | 2 | 16 |
| 2 Ru/TiO2 (DP) | 46 | 63 | 19 | 2 | 16 |
| 2 Ru/TiO2 (DP) a | 44 | 63 | 19 | 2 | 16 |
| 2 Ru/TiO2 (DP) b | 42 | 59 | 22 | 2 | 17 |
| 5 Ru/TiO2 (DP) | 44 | 58 | 17 | 4 | 21 |
| 5 Ru/TiO2 (IM) | 31 | 49 | 24 | 2 | 15 |
| 7 Ru/TiO2 (DP) | 40 | 64 | 18 | 7 | 11 |
| Run | Conversion | Selectivity (%) a | CMB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| at 24 h (%) | GA | TA | GLA | OA | DHA | HPA | FA | ||
| Standard | 18.0 | 64.7 | 1.1 | 2.7 | 0.1 | 30.2 | 1.2 | - | 95.2 |
| Reuse 1 | 18.4 | 60.3 | 0.6 | 0.6 | 0.1 | 35.0 | 1.0 | 1.4 | 88.2 |
| Reuse 2 | 7.7 | 49.7 | 3.1 | 1.8 | 0.5 | 42.9 | 1.1 | 0.9 | 94.7 |
| Carbonyl Compound a | Catalyst | Time (h) | Selectivity 5-Members/6-Members | Yield (%) |
|---|---|---|---|---|
| Benzaldehyde | GR | 2 | 63/37 | 94 |
| Benzaldehyde | GR-SO3H | 2 | 33/67 | 49 |
| Anisaldehyde | GR | 2 | 63/37 | 99 |
| Trans Cinnamaldehyde | GR | 2 | 66/34 | 89 |
| Furfural | GR | 2 | 68/32 | 85 b |
| Acetone | GR | 2 | >99/<1 | 76 |
| Acetone | GR | 2 | >99/<1 | 85 c |
| Acetone | GR-SO3H | 14 | >99/<1 | 59 c |
| Acetone | - | 14 | - | 0 c |
| Acetophenone | GR | 2 | >99/<1 | 25 |
| Acetophenone | GR | 2 | >99/<1 | 72 c |
| Cyclopentanone | GR | 2 | >99/<1 | 99 |
| Cyclohexanone | GR | 2 | >99/<1 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlucci, C. A Focus on the Transformation Processes for the Valorization of Glycerol Derived from the Production Cycle of Biofuels. Catalysts 2021, 11, 280. https://doi.org/10.3390/catal11020280
Carlucci C. A Focus on the Transformation Processes for the Valorization of Glycerol Derived from the Production Cycle of Biofuels. Catalysts. 2021; 11(2):280. https://doi.org/10.3390/catal11020280
Chicago/Turabian StyleCarlucci, Claudia. 2021. "A Focus on the Transformation Processes for the Valorization of Glycerol Derived from the Production Cycle of Biofuels" Catalysts 11, no. 2: 280. https://doi.org/10.3390/catal11020280