A Novel Synthesizing Strategy of 3D Cose2 Porous Hollow Flowers for High Performance Lithium–Sulfur Batteries
Abstract
:1. Introduction
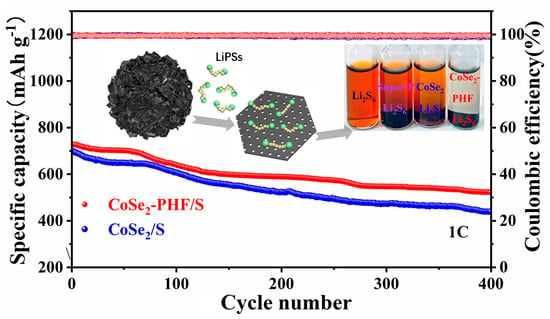
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of CoAl-LDH Precursors
3.2. Synthesis of CoSe2-PHF, CoSe2, CoS2 and Co3O4
3.3. Synthesis of CoSe2-PHF/S, CoS2/S and Co3O4/S and CoSe2/S Composite
3.4. Visualized Adsorption Test of Polysulfides
3.5. Electrochemical Measurement
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Yang, W.; Liu, J.; Zhou, Y. Decorating CoSe2 hollow nanospheres on reduced graphene oxide as advanced sulfur host material for performance enhanced lithium-sulfur batteries. Nano Res. 2019, 12, 2743–2748. [Google Scholar]
- Rana, M.; Ahad, S.A.; Li, M.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Review on areal capacities and long-term cycling performances of lithium sulfur battery at high sulfur loading. Energy Storage Mater. 2019, 18, 289–310. [Google Scholar]
- Yu, J.; Khan, S.A.; Zhao, D.; Li, L.; Wu, Z.; Niu, X.; Chen, S. Nitrogen and iron codoped porous carbon polyhedra for effectively confining polysulfides and efficiently catalyzing their conversion in lithium–sulfur batteries. Sustain. Energy Fuels 2020, 4, 5215–5222. [Google Scholar]
- Zhao, J.; Zhao, D.; Li, L.; Zhou, L.; Liang, X.; Wu, Z.; Jiang, Z.-J. Defect-rich, mesoporous cobalt sulfide hexagonal nanosheets as superior sulfur hosts for high-rate, long-cycle rechargeable lithium–sulfur batteries. J. Phys. Chem. C 2020, 124, 12259–12268. [Google Scholar]
- Zeng, S.; Li, L.; Yu, J.; Wang, N.; Chen, S. Highly crosslinked organosulfur copolymer nanosheets with abundant mesopores as cathode materials for efficient lithium-sulfur batteries. Electrochim. Acta 2018, 263, 53–59. [Google Scholar]
- Tsao, Y.; Lee, M.; Miller, E.C.; Gao, G.; Park, J.; Chen, S.; Katsumata, T.; Tran, H.; Wang, L.-W.; Toney, M.F.; et al. Designing a quinone-based redox mediator to facilitate Li2S oxidation in Li–S batteries. Joule 2019, 3, 872–884. [Google Scholar]
- Li, T.; Bai, X.; Gulzar, U.; Bai, Y.J.; Capiglia, C.; Deng, W.; Zhou, X.; Liu, Z.; Feng, Z.; Proietti Zaccaria, R. A comprehensive understanding of lithium–sulfur battery technology. Adv. Funct. Mater. 2019, 29, 1901730. [Google Scholar]
- Wang, X.L.; Chen, J.; Jin, B.; Jiang, Q.; Jin, E.M.; Jeong, S.M. Electrochemical performance of electrospun lotus-root-structure porous multichannel carbon nanotubes for lithium–sulfur battery applications. J. Electroanal. Chem. 2020, 878, 114564. [Google Scholar]
- Xu, N.; Qian, T.; Liu, X.; Liu, J.; Chen, Y.; Yan, C. Greatly suppressed shuttle effect for improved lithium sulfur battery performance through short chain intermediates. Nano Lett. 2017, 17, 538–543. [Google Scholar]
- Xie, J.; Li, B.Q.; Peng, H.J.; Song, Y.W.; Zhao, M.; Chen, X.; Zhang, Q.; Huang, J.Q. Implanting atomic cobalt within mesoporous carbon toward highly stable lithium–sulfur batteries. Adv. Mater. 2019, 31, 1903813. [Google Scholar]
- Zhao, M.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. A perspective toward practical lithium–sulfur batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar]
- Shaibani, M.; Sharifzadeh Mirshekarloo, M.; Singh, R.; Easton, C.D.; Cooray, M.C.D.; Eshraghi, N.; Abendroth, T.; Dörfler, S.; Althues, H.; Kaskel, S.; et al. Expansion-tolerant architectures for stable cycling of ultrahigh-loading sulfur cathodes in lithium-sulfur batteries. Sci. Adv. 2020, 6, eaay2757. [Google Scholar] [CrossRef] [Green Version]
- Gueon, D.; Hwang, J.T.; Yang, S.B.; Cho, E.; Sohn, K.; Yang, D.K.; Moon, J.H. Spherical macroporous carbon nanotube particles with ultrahigh sulfur loading for lithium-sulfur battery cathodes. ACS Nano 2018, 12, 226–233. [Google Scholar]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2011, 50, 5904–5908. [Google Scholar]
- Li, M.; Carter, R.; Douglas, A.; Oakes, L.; Pint, C.L. Sulfur vapor-infiltrated 3D carbon nanotube foam for binder-free high areal capacity lithium-sulfur battery composite cathodes. ACS Nano 2017, 11, 4877–4884. [Google Scholar]
- Li, S.; Jin, B.; Zhai, X.; Li, H.; Jiang, Q. Review of carbon materials for lithium-sulfur batteries. ChemistrySelect 2018, 3, 2245–2260. [Google Scholar]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes. Angew. Chem. Int. Ed. Engl. 2015, 54, 4325–4329. [Google Scholar]
- Zhang, Z.; Kong, L.-L.; Liu, S.; Li, G.-R.; Gao, X.-P. A high-efficiency sulfur/carbon composite based on 3D graphene nanosheet@carbon nanotube matrix as cathode for lithium-sulfur battery. Adv. Energy Mater. 2017, 7, 1602543. [Google Scholar]
- Zeng, S.; Li, L.; Xie, L.; Zhao, D.; Zhou, N.; Wang, N.; Chen, S. Graphene-supported highly crosslinked organosulfur nanoparticles as cathode materials for high-rate, long-life lithium-sulfur battery. Carbon 2017, 122, 106–113. [Google Scholar]
- Zheng, C.; Niu, S.; Lv, W.; Zhou, G.; Li, J.; Fan, S.; Deng, Y.; Pan, Z.; Li, B.; Kang, F.; et al. Propelling polysulfides transformation for high-rate and long-life lithium–sulfur batteries. Nano Energy 2017, 33, 306–312. [Google Scholar]
- Kong, L.; Li, B.-Q.; Peng, H.-J.; Zhang, R.; Xie, J.; Huang, J.-Q.; Zhang, Q. Porphyrin-derived graphene-based nanosheets enabling strong polysulfide chemisorption and rapid kinetics in lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1800849. [Google Scholar]
- Zhou, G.; Sun, J.; Jin, Y.; Chen, W.; Zu, C.; Zhang, R.; Qiu, Y.; Zhao, J.; Zhuo, D.; Liu, Y.; et al. Sulfiphilic nickel phosphosulfide enabled Li2S impregnation in 3D graphene cages for Li–S batteries. Adv. Mater. 2017, 29, 1603366. [Google Scholar]
- Fan, X.; Sun, W.; Meng, F.; Xing, A.; Liu, J. Advanced chemical strategies for lithium–sulfur batteries: A review. Green Energy Environ. 2018, 3, 2–19. [Google Scholar]
- Jiang, G.; Xu, F.; Yang, S.; Wu, J.; Wei, B.; Wang, H. Mesoporous, conductive molybdenum nitride as efficient sulfur hosts for high-performance lithium-sulfur batteries. J. Power Sources 2018, 395, 77–84. [Google Scholar]
- Liu, Y.T.; Han, D.D.; Wang, L.; Li, G.R.; Liu, S.; Gao, X.P. NiCo2O4 nanofibers as carbon-free sulfur immobilizer to fabricate sulfur-based composite with high volumetric capacity for lithium–sulfur battery. Adv. Energy Mater. 2019, 9, 1803477. [Google Scholar]
- Pu, J.; Shen, Z.; Zheng, J.; Wu, W.; Zhu, C.; Zhou, Q.; Zhang, H.; Pan, F. Multifunctional Co3S4@sulfur nanotubes for enhanced lithium-sulfur battery performance. Nano Energy 2017, 37, 7–14. [Google Scholar]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D metal carbides and nitrides (MXenes) as high-performance electrode materials for lithium-based batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar]
- Zhao, M.; Peng, H.J.; Zhang, Z.W.; Li, B.Q.; Chen, X.; Xie, J.; Chen, X.; Wei, J.Y.; Zhang, Q.; Huang, J.Q. activating inert metallic compounds for high-rate lithium-sulfur batteries through in situ etching of extrinsic metal. Angew. Chem. Int. Ed. Engl. 2019, 58, 3779–3783. [Google Scholar]
- Chen, L.; Li, X.; Xu, Y. Recent advances of polar transition-metal sulfides host materials for advanced lithium–sulfur batteries. Funct. Mater. Lett. 2018, 11, 1840010. [Google Scholar]
- Guo, T.; Song, Y.; Sun, Z.; Wu, Y.; Xia, Y.; Li, Y.; Sun, J.; Jiang, K.; Dou, S.; Sun, J. Bio-templated formation of defect-abundant VS2 as a bifunctional material toward high-performance hydrogen evolution reactions and lithium−sulfur batteries. J. Energy Chem. 2020, 42, 34–42. [Google Scholar]
- Li, Y.; Jiang, T.; Yang, H.; Lei, D.; Deng, X.; Hao, C.; Zhang, F.; Guo, J. A heterostuctured Co3S4/MnS nanotube array as a catalytic sulfur host for lithium–sulfur batteries. Electrochim. Acta 2020, 330, 135311. [Google Scholar]
- Zhang, K.; Chen, F.; Pan, H.; Wang, L.; Wang, D.; Jiang, Y.; Wang, L.; Qian, Y. Study on the effect of transition metal sulfide in lithium–sulfur battery. Inorg. Chem. Front. 2019, 6, 477–481. [Google Scholar]
- Zhou, G.; Zhao, S.; Wang, T.; Yang, S.Z.; Johannessen, B.; Chen, H.; Liu, C.; Ye, Y.; Wu, Y.; Peng, Y.; et al. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li–S batteries. Nano Lett. 2020, 20, 1252–1261. [Google Scholar]
- Choi, S.H.; Kang, Y.C. Fullerene-like MoSe2 nanoparticles-embedded CNT balls with excellent structural stability for highly reversible sodium-ion storage. Nanoscale 2016, 8, 4209–4216. [Google Scholar]
- Zhang, C.; Biendicho, J.J.; Zhang, T.; Du, R.; Li, J.; Yang, X.; Arbiol, J.; Zhou, Y.; Morante, J.R.; Cabot, A. Combined high catalytic activity and efficient polar tubular nanostructure in urchin-like metallic NiCo2Se4 for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 2019, 29, 1903842. [Google Scholar]
- Zhu, Y.; Huang, Z.; Hu, Z.; Xi, L.; Ji, X.; Liu, Y. 3D interconnected ultrathin cobalt selenide nanosheets as cathode materials for hybrid supercapacitors. Electrochim. Acta 2018, 269, 30–37. [Google Scholar]
- Zhang, H.; Yang, B.; Wu, X.; Li, Z.; Lei, L.; Zhang, X. Polymorphic CoSe2 with mixed orthorhombic and cubic phases for highly efficient hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 1772–1779. [Google Scholar]
- Liu, Y.; Cheng, H.; Lyu, M.; Fan, S.; Liu, Q.; Zhang, W.; Zhi, Y.; Wang, C.; Xiao, C.; Wei, S.; et al. Low overpotential in vacancy-rich ultrathin CoSe2 nanosheets for water oxidation. J. Am. Chem. Soc. 2014, 136, 15670–15675. [Google Scholar]
- Chen, T.; Li, S.; Wen, J.; Gui, P.; Guo, Y.; Guan, C.; Liu, J.; Fang, G. Rational construction of hollow core-branch CoSe2 nanoarrays for high-performance asymmetric supercapacitor and efficient oxygen evolution. Small 2017, 14, 1700979. [Google Scholar]
- Kim, J.K.; Park, G.D.; Kim, J.H.; Park, S.K.; Kang, Y.C. Rational design and synthesis of extremely efficient macroporous CoSe2-CNT composite microspheres for hydrogen evolution reaction. Small 2017, 13, 1700068. [Google Scholar]
- Han, S.; Chang, X.; Wu, D.; Chen, H.; Chen, D.; Liu, P.; Huang, T.; Jiang, X.; Huang, Q.; Lin, H. Hierarchically porous cobalt aluminum layered double hydroxide flowers with enhanced capacitance performances. J. Mater. Sci. 2017, 52, 6081–6092. [Google Scholar]
- Jing, C.; Liu, X.; Yao, H.; Yan, P.; Zhao, G.; Bai, X.; Dong, B.; Dong, F.; Li, S.; Zhang, Y. Phase and morphology evolution of CoAl-LDH nanosheets towards advanced supercapacitor applications. CrystEngComm 2019, 21, 4934–4942. [Google Scholar]
- Zhang, Y.; Du, D.; Li, X.; Sun, H.; Li, L.; Bai, P.; Xing, W.; Xue, Q.; Yan, Z. Electrostatic self-assembly of sandwich-like CoAl-LDH/polypyrrole/graphene nanocomposites with enhanced capacitive performance. ACS Appl. Mater. Interfaces 2017, 9, 31699–31709. [Google Scholar]
- Li, Y.; Wu., J.; Zhang, B.; Wang, W.; Zhang, G.; Seh, Z.W.; Zhang, N.; Sun, J.; Huang, L.; Jiang, J.; et al. Fast conversion and controlled deposition of lithium (poly) sulfides in lithium-sulfur batteries using high-loading cobalt single atoms. Energy Storage Mater. 2020, 30, 250–259. [Google Scholar]
- Sun, Z.; Vijay, S.; Heenen, H.H.; Eng, A.Y.S.; Tu, W.; Zhao, Y.; Koh, S.W.; Gao, P.; Seh, Z.W.; Chan, K.; et al. Catalytic polysulfide conversion and physiochemical confinement for lithium–sulfur batteries. Adv. Energy Mater. 2020, 10, 1904010. [Google Scholar]
- Wu, X.; Liu, N.; Wang, M.; Qiu, Y.; Guan, B.; Tian, D.; Guo, Z.; Fan, L.; Zhang, N. A class of catalysts of BiOX (X = Cl, Br, I) for anchoring polysulfides and accelerating redox reaction in lithium sulfur batteries. ACS Nano 2019, 13, 13109–13115. [Google Scholar]
- Li, R.; Peng, H.; Wu, Q.; Zhou, X.; He, J.; Shen, H.; Yang, M.; Li, C. Sandwich-like Catalyst–Carbon–Catalyst Trilayer Structure as a Compact 2D Host for Highly Stable Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 12129–12138. [Google Scholar]
- Wu, F.; Qian, J.; Chen, R.; Zhao, T.; Xu, R.; Ye, Y.; Li, W.; Li, L.; Lu, J.; Amine, K. Sulfur cathode based on layered carbon matrix for high-performance Li–S batteries. Nano Energy 2015, 12, 742–749. [Google Scholar]
- Jiang, Y.; Liu, H.; Tan, X.; Guo, L.; Zhang, J.; Liu, S.; Guo, Y.; Zhang, J.; Wang, H.; Chu, W. Monoclinic ZIF-8 nanosheet-derived 2D carbon nanosheets as sulfur immobilizer for high-performance lithium sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 25239–25249. [Google Scholar]
- Su, D.; Cortie, M.; Fan, H.; Wang, G. Prussian blue nanocubes with an open framework structure coated with PEDOT as high-capacity cathodes for lithium-sulfur batteries. Adv. Mater. 2017, 29, 1700587. [Google Scholar]
- Xu, J.; Zhang, W.; Chen, Y.; Fan, H.; Su, D.; Wang, G. MOF-derived porous N–Co3O4@N–C nanododecahedra wrapped with reduced graphene oxide as a high capacity cathode for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 2797–2807. [Google Scholar]








| Samples | SBET (m2·g−1) | Total Pore Volume (cm3·g−1) | Mean Pore Diameter (nm) |
|---|---|---|---|
| CoAl-LDH | 19.515 | 0.058 | 5.012 |
| Co-PHF | 40.177 | 0.169 | 17.296 |
| CoSe2-PHF | 31.986 | 0.108 | 15.310 |
| CoSe2 | 18.884 | 0.063 | 8.690 |
| CoS2 | 19.659 | 0.065 | 9.114 |
| Co3O4 | 22.665 | 0.059 | 8.796 |
| Samples | σ (S cm−1) |
|---|---|
| S [5] | 5.00 × 10−30 |
| Co3O4 | 2.27 × 10−5 |
| CoS2 | 1.90 × 10−5 |
| CoSe2 | 2.57 × 10−3 |
| CoSe2-PHF | 1.49 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Wu, Q.; Che, Z.; Fan, B.; Zhao, D.; Wang, S.; Han, A.; Li, L. A Novel Synthesizing Strategy of 3D Cose2 Porous Hollow Flowers for High Performance Lithium–Sulfur Batteries. Catalysts 2021, 11, 273. https://doi.org/10.3390/catal11020273
Xu W, Wu Q, Che Z, Fan B, Zhao D, Wang S, Han A, Li L. A Novel Synthesizing Strategy of 3D Cose2 Porous Hollow Flowers for High Performance Lithium–Sulfur Batteries. Catalysts. 2021; 11(2):273. https://doi.org/10.3390/catal11020273
Chicago/Turabian StyleXu, Wei, Qikai Wu, Zhongmei Che, Bin Fan, Dengke Zhao, Shuai Wang, Aixia Han, and Ligui Li. 2021. "A Novel Synthesizing Strategy of 3D Cose2 Porous Hollow Flowers for High Performance Lithium–Sulfur Batteries" Catalysts 11, no. 2: 273. https://doi.org/10.3390/catal11020273