Technological Aspects of Highly Selective Synthesis of Allyloxyalcohols—New, Greener, Productive Methods
Abstract
:1. Introduction
- •
- the monoallyl ethers of ethylene or propylene glycols or polyglycols, obtained via the alkoxylation of AA by ethylene oxide or propylene oxide; of course, this synthetic way solely leads to allyloxyalcohols but it is limited to only one type of them containing one allyl and one hydroxyl group in the molecule;
- •
- the allyl ethers of partially substituted polyols such as neopentl glycol, glycerol, pentaerythritol, and trimethylolpropane, easily produced by the partial etherification of polyols with allylating agents (O-allylation reaction), this is much more universal and more commonly used in the case of multifunctional, smart, and environmentally friendly bio-renewable allyl ethers (Scheme 2).
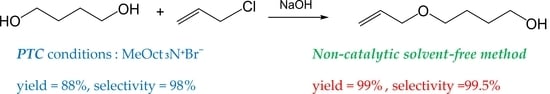
2. Results and Discussion
2.1. Mono-O-Allylation Reaction under PTC Conditions
2.2. Mono-O-Allylation Reaction under Solvent-Free Non-Catalytic Conditions
3. Materials and Methods
3.1. General
- •
- 4-alliloxybutan-1-ol: bp. 62 °C/0.5 mmHg; 1H NMR (400 Hz, CDCl3): 5.82–5.74 (m, 1H, CH2=CH-), 5.15–5.12 (d, J = 17.2 Hz, 1H(E), CH2=CH-), 5.08–5.03 (d, J = 10.4 Hz, 1H(Z), CH2=CH-), 4.30 (s, 1H, -OH), 3.85 (d, J = 5.0 Hz, 2H, =CH-CH2-O-), 3.47 (t, J = 11.4 Hz, 2H, -CH2-OH), 3.33 (t, J = 12.3 Hz, 2H, -O-CH2-), 1.56–1.50 (m, 4H, -CH2-); 13C NMR (400 Hz, CDCl3) 135.5 (CH2=CH-), 117.1 (CH2=CH-), 72.3 (=CH-CH2-O-), 70.6 (-CH2-O-CH2-), 62.8 (-CH2-OH), 29.8, 26.7 (-CH2-CH2-); MS (m/q, int (%)): 130 (M+ 0.03%); 89 (13); 73 (54); 71 (84); 58 (26); 57 (44); 55 (77); 43 (52); 41 (100); 39 (39); 31 (38); 29 (18); refractive index (24.5 °C): 1.4400, density (20 °C): 0.9288 g/cm3;
- •
- 1,4-bisalliloxybutane: bp. 52 °C/0.5 mmHg; 1H NMR (400 Hz, CDCl3): 5.82–5.76 (m, 2H, CH2=CH-), 5.18–5.13 (d, J = 17.3 Hz, 2H(E), CH2=CH-), 5.05–5.01 (d, J = 10.4 Hz, 2H(Z), CH2=CH-), 3.84 (m, 4H, =CH-CH2-O-), 3.33 (m, 4H, -O-CH2-), 1.65 (4H, -CH2-); 13C NMR (400 Hz, CDCl3) 135.1 (CH2=CH-), 116.3 (CH2=CH-), 71.6 (=CH-CH2-O-), 70.6 (-CH2-O-CH2-), 26.4 (-CH2-CH2-); MS (m/q, int (%)): 170 (M+ 0.002%); 129 (11); 113 (12); 71 (100); 55 (19); 43 (17); 41 (78); 39 (19); 29 (7); refractive index (24.5 °C): 1.4360, density (20 °C): 0.8805 g/cm3.
3.2. The Procedure of O-Allylation Reaction under PTC Conditions
3.3. The Procedure of O-Allylation Reaction under Solvent-Free Non-Catalytic Conditions
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, 6th ed.; Wiley-Interscience: New York, NY, USA, 2007. [Google Scholar]
- Godleski, S.A. Comprehensive Organic Synthesis; Pergamon: Oxford, UK, 1991; Volume 4, pp. 586–661. [Google Scholar]
- Krähling, L.; Krey, J.; Jakobson, G.; Grolig, J.; Miksche, L. Allyl Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Schildknecht, C.E. Allyl Compounds and Their Polymers (Including Polyolefins); Wiley-Interscience: New York, NY, USA; London, UK, 1973. [Google Scholar]
- Jensen, E.S.; Gatenholm, P.; Nanguneri, S.R.; Mathias, L. Effect of chemical structure of allyl ethers on polymerization and properties of multifunctional acrylate systems. J. Appl. Polym. Sci. 1991, 42, 2681. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, X.; Zeng, Z.; Yang, J.; Chen, Y. Allyl ether-modified unsaturated polyesters for UV/air dual-curable coatings. I: Synthesis and characterization of the oligomers and their cured films. J. Appl. Polym. Sci. 2004, 92, 2765–2770. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Rokicki, G.; Szymańska, E. Unsaturated Polyester Resins with Different Allyl Ethers as Crosslinking Built-In Monomers. J. Appl. Polym. Sci. 1998, 70, 2031–2039. [Google Scholar] [CrossRef]
- Crivello, J.V.; Jo, K.D. Propenyl Ethers I. The Synthesis of Propenyl Ether Monomers. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1473–1482. [Google Scholar] [CrossRef]
- Krompiec, S.; Kuźnik, N.; Urbala, M.; Rzepa, J. Isomerization of alkyl allyl and allyl silyl ethers catalyzed by ruthenium complexes. J. Mol. Catal. A Chem. 2006, 248, 98–209. [Google Scholar] [CrossRef]
- Urbala, M. The effectiveness of ruthenium(II) complexes and ruthenium trichloride as pre-catalysts in solvent-free isomerization of model alkyl allyl ether. Appl. Catal. A Gen. 2010, 377, 27–34. [Google Scholar] [CrossRef]
- Urbala, M.; Krompiec, S.; Penkala, M.; Danikiewicz, W.; Grela, M. Solvent-free Ru-catalyzed isomerization of allyloxyalcohols: Methods for highly selective synthesis of 1-propenyloxyalcohols. Appl. Catal. A Gen. 2013, 451, 101–111. [Google Scholar] [CrossRef]
- Urbala, M. Solvent-free [Ru]-catalyzed isomerization of allyl glycidyl ether: The scope, effectiveness and recycling of catalysts, and exothermal effect. Appl. Catal. A Gen. 2015, 505, 382–393. [Google Scholar] [CrossRef]
- Urbala, M. Highly Productive Synthesis of 1-Propenyloxybutan-1-ol Under Solvent-Free Homogeneous Ruthenium Catalyst Conditions. Catalysts 2020, 10, 1409. [Google Scholar] [CrossRef]
- Bouvet-Marchand, A.; Chatard, C.; Graillot, A.; Boutevin, G.; Loubat, C.; Grossob, D. Influence of experimental parameters on the side reactions of hydrosilylation of allyl polyethers studied by a fractional factorial design. React. Chem. Eng. 2018, 3, 696–706. [Google Scholar] [CrossRef]
- Konuraya, O.; Fernández-Francosa, X.; Ramisa, X.; Serrab, A. New allyl-functional catalytic comonomers for sequential thiol-Michael and radical thiolene reactions. Polymer 2018, 138, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, C.; Simpson, N.; Malkoch, M.; Johansson, M.; Malmström, E. Synthesis and thiol-ene photopolymerization of allyl-ether functionalized dendrimers. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1339–1348. [Google Scholar] [CrossRef]
- Resetco, C.; Hendriks, B.; Badi, N.; Du Prez, F. Thiol–ene chemistry for polymer coatings and surface modification—Building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Dutta, B.; Clarke, R.; Raman, S.; Shaffer, T.D.; Achola, L.; Nandi, P.; Suib, S.L. Lithium promoted mesoporous manganese oxide catalyzed oxidation of allyl ethers. Nat. Commun. 2019, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Sutter, M.; Da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef]
- Rao, B.V.S.K.; Gangadhar, A.; Subbarao, R.; Lakshminarayana, G.A. Facile synthesis of rac-1-O-alkylglycerols. Org. Prep. Proced. Int. 1991, 23, 119–122. [Google Scholar] [CrossRef]
- Shing, T.K.M.; Tai, V.W.F.; Tam, E.K.W. Practical and Rapid Vicinal Hydroxylation of Alkenes by Catalytic Ruthenium Tetraoxide. Angewandte Chemie 1994, 33, 2312–2313. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Zhang, X.-L.; Sharpless, K.B. Asymmetric dihydroxylation of aryl allyl ethers. Tetrahedron Lett. 1993, 34, 2267–2270. [Google Scholar] [CrossRef]
- Johansson, M.; Malmström, E.; Hult, A. Synthesis, characterization, and curing of hyperbranched allyl ether–maleate functional ester resins. J. Polym. Sci. A Polym. Chem. 1993, 31, 619–624. [Google Scholar] [CrossRef]
- Stacy, M.T.; Nilsson, C.; Malmström, E.; Johansson, M. Thiolene networks and reactive surfaces via photoinduced polymerization of allyl ether functional hyperbranched polymers. Prog. Org. Coat. 2010, 67, 348–355. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Ferris, C.; de Paz, M.V.; Galbis, J.A. Synthesis of functional sugar-based polyurethanes. Macromol. Chem. Phys. 2012, 213, 480–488. [Google Scholar] [CrossRef]
- Galbis, J.A.; García-Martín, M.G.; de Paz, M.V.; Galbis, E. Synthetic polymers from sugar-based monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.L.; Ionescu, M.; Wan, X.; Upshaw, T. Biobased Aromatic-Aliphatic Polyols by Thiol-Ene Reactions of Propoxylated Mercaptanized Cardanol. J. Renew. Mater. 2018, 6, 630–641. [Google Scholar] [CrossRef]
- Besse, V.; Camara, F.; Voirin, C.; Auvergne, R.; Caillol, S.; Boutevina, B. Synthesis and applications of unsaturated cyclocarbonates. Polym. Chem. 2013, 4, 4545–4561. [Google Scholar] [CrossRef]
- Mindemark, J.; Imholt, L.; Montero, J.; Brandell, D. Allyl ethers as combined plasticizing and crosslinkable side groups in polycarbonate-based polymer electrolytes for solid-state Li batteries. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2128–2135. [Google Scholar] [CrossRef]
- Olsén, P.; Odelius, K.; Albertsson, A.-C. Ring-Closing Depolymerization: A Powerful Tool for Synthesizing the Allyloxy-Functionalized Six-Membered Aliphatic Carbonate Monomer 2-Allyloxymethyl-2-ethyltrimethylene Carbonate. Macromolecules 2014, 47, 6189–6195. [Google Scholar] [CrossRef]
- Niemczyk, E.M.; Gomez-Lopez, A.; Haler, J.R.N.; Frache, G.; Sardon, H.; Quintana, R. Insights on the Atmospheric-Pressure Plasma-Induced Free-Radical Polymerization of Allyl Ether Cyclic Carbonate Liquid Layers. Polymers 2021, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- Urbala, M.; Antoszczyszyn, M. The synthesis of allyl ether functionalized siloxane monomers under ultrasonic irradiation at ambient conditions. Ultrason. Sonochem. 2004, 11, 409. [Google Scholar] [CrossRef]
- Kuciński, K.; Hreczycho, G. Synthesis of novel bifunctional organosilicon dendrons via platinum-catalyzed hydrosilylation. Inorg. Chim. Acta 2017, 461, 233–238. [Google Scholar] [CrossRef]
- Skrodzki, M.; Zaranek, M.; Witomska, S.; Pawluc, P. Direct Dehydrogenative Coupling of Alcohols with Hydrosilanes Promoted by Sodium tri(sec-butyl)borohydride. Catalysts 2018, 8, 618. [Google Scholar] [CrossRef] [Green Version]
- Global Epichlorohydrin (ECH) Industry 2020. Available online: https://www.globenewswire.com/news-release/2020/08/08/2075328/0/en/Global-Epichlorohydrin-ECH-Industry.html (accessed on 13 September 2021).
- Obermeier, B.; Frey, H. Poly(ethylene glycol-co-allyl glycidyl ether)s: A PEG-Based Modular Synthetic Platform for Multiple Bioconjugation. Bioconjugate Chem. 2011, 22, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Ikeda, M.; Miura, M.; Nomura, M. Palladium-Catalyzed Etherification of Allyl Alcohols Using Phenols in the Presence of Titanium(IV) Isopropoxide. J. Org. Chem. 1997, 62, 4877. [Google Scholar] [CrossRef]
- Saburi, H.; Tanaka, S.; Kitamura, M. Catalytic Dehydrative Allylation of Alcohols. Angewandte Chemie 2005, 117, 1758–1760. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hirabayashi, T.; Sakaguchi, S.; Ishii, Y. Allylation of Alcohols and Carboxylic Acids with Allyl Acetate Catalyzed by [Ir(cod)2]+BF4− Complex. J. Org. Chem. 2004, 69, 3474–3477. [Google Scholar] [CrossRef]
- Tang, H.; Tian, Y.B.; Cui, H.; Li, R.Z.; Zhang, X.; Niu, D. Site-switchable mono-O-allylation of polyols. Nat. Commun. 2020, 11, 5681. [Google Scholar] [CrossRef] [PubMed]
- Kon, Y.; Fujitani, T.; Nakashima, T.; Murayamabc, T.; Ueda, W. Versatile etherification of alcohols with allyl alcohol by a titanium oxide-supported molybdenum oxide catalyst: Gradual generation from titanium oxide and molybdenum oxide. Catal. Sci. Technol. 2018, 8, 4618–4625. [Google Scholar] [CrossRef]
- Antoszczyszyn, M.; Janus, E.; Urbala, M. Synthesis of mono- and diallyl ethers of 1,4-dihydroxybutane, Z-1,4-dihydroxy-2-butene and 1,4-dihydroxy-2-butyne. Pol. J. Appl. Chem. 1999, 43, 77–83. [Google Scholar]
- Martysz, D.; Urbala, M.; Antoszczyszyn, M.; Pilawka, R. l-Propenyl ethers of butanediol as effective modifiers of UV-cured epoxy coatings in cationic polymerization. Polimery 2002, 11–12, 849–851. [Google Scholar] [CrossRef]
- Martysz, D.; Antoszczyszyn, M.; Urbala, M.; Krompiec, S.; Fabrycy, E. Synthesis of 1-propenyl ethers and their using as modifiers of UV-cured coatings in radical and cationic polymerization. Prog. Org. Coat. 2003, 46, 302–311. [Google Scholar] [CrossRef]
- Czech, Z.; Urbala, M. UV-crosslinked acrylic pressure-sensitive adhesive systems containing unsaturated ethers. Polimery 2007, 6, 438–442. [Google Scholar] [CrossRef] [Green Version]
- The global market of 1,4-butanediol. Available online: https://www.grandviewresearch.com/industry-analysis/1-4-butanediol-market (accessed on 13 September 2021).
- Rosales-Calderon, O.; Arantes, V.A. Review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef] [Green Version]
- Harmata, M.; Rashatasakhon, P.; Barnes, C.L. Intramolecular [4 + 3] cycloadditions—Stereochemical issues in the cycloaddition reactions of cyclopentenyl cations—A synthesis of (+)-dactylol. Can. J. Chem. 2006, 84, 1456–1469. [Google Scholar] [CrossRef]
- Crivello, J.V.; Yang, B.; Kim, W.-G. Chemoselective hydrosilations. I. Synthesis and photopolymerization of 1-propenyl ether functionalized siloxanes. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2415–2423. [Google Scholar] [CrossRef]
- Halpern, M. Phase-Transfer Catalysis. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar] [CrossRef]
- Mąkosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Mąkosza, M.; Fedoryński, M. Phase Transfer Catalysis. Catal. Rev. Sci. Eng. 2003, 45, 321–367. [Google Scholar] [CrossRef]
- Mąkosza, M.; Fedoryński, M. Interfacial Processes—The Key Steps of Phase Transfer Catalyzed Reactions. Catalysts 2020, 10, 1436. [Google Scholar] [CrossRef]
- Albanese, D. Liquid–Liquid Phase Transfer Catalysis: Basic Principles and Synthetic Applications. Catal. Rev. Sci. Eng. 2003, 45, 369–395. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Morozova, N.V.; Lebedeva, N.N.; Panicheva, L.P. Alkylation of diols under phase transfer conditions: The influence of the nature of the substrate, alkylating agent, and organic solvent. Petroleum Chem. 2009, 49, 381–384. [Google Scholar] [CrossRef]
- Saberian, M.; Naghash, H.J. Synthesis of allyl ether and benzyl methacrylate functionalized silane monomers for application in acrylate/montmorillonite nanocomposite emulsion. J. Adhes. Sci. Technol. 2020, 34, 153–166. [Google Scholar] [CrossRef]
- Nichols, P.L.; Hamilton, R.M.; Smith, L.T.; Yanovsky, E. Allyl Ether of Starch. Ind. Eng. Chem. 1945, 37, 201–202. [Google Scholar] [CrossRef]








| Entry | 1 (mol%) | NaOH:BDO Molar Ratio | NaOH(aq) Conc. (%) | I Step Time (min)/t (°C) | II Step Time (h)/50 °C/So | SABO (%) | YABO (%) | YAA/YAE (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2.5 | 35 | 30/90 | 10/T | 95 | 80 | 3/15 |
| 2 | 30/50 | 97 | 68 | 8/5 | ||||
| 3 | 1 | 0.6 | 50 | 30/90 | 10/T | 97 | 58 | 2/2 |
| 4 | 30/50 | 10/T | 98 | 51 | 1.5/1 | |||
| 5 | 1 | 1 | 50 | 30/90 | 6/T | 84 | 81 | 2/2 |
| 6 | 30/50 | 6/T | 96 | 72 | 1.5/1 | |||
| 7 | 30/50 | 8/T | 86 | 87 | 2/1.3 | |||
| 8 | 15/50 | 8/T | 88 | 84 | 1/1 | |||
| 9 | - | 8/T | 92 | 63 | 2/1 | |||
| 10 | 0.1 | 1 | 50 | 15/50 | 6/T | 98 | 54 | 2/1 |
| 11 | 8/T | 96 | 61 | ~2/1.5 | ||||
| 12 | 10/T | 94 | 65 | 2.5/2 | ||||
| 13 | 6/C | ~98 | 57 | traces | ||||
| 14 | 8/C | 95 | 66 | 1/1 | ||||
| 15 | 10/C | 95 | 69 | 1.2/1 | ||||
| 16 | - | 1 | 50 | 15/50 | 8/C | 100 | 37 | 1.5/1 |
| 17 | 48/C | 99 | 88 | 4/3 | ||||
| 18 2 | 6/C | 97 | 47 | 1/1 | ||||
| 19 3 | 6/C | 95 | 59 | traces |
| Entry | NaOH:AC Molar Ratio | I Step Time (min.)/50 °C | II Step Time (h)/50 °C | αAC (%) | SABO (%) | YABO (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 50 | 3 | 91 | 98.9 | 90 |
| 2 | 1.05 | 75 | 2.5 | 97 | 99.0 | 96 |
| 3 | 1.1 | 90 | 2 | 100 | 99.2 | 99.2 |
| 4 | 1.1 | 80 | 2 | 100 | 99.0 | 99 |
| 5 3 | 1.1 | 100 | 2.5 | ~100 | 99.3 | 99.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbala, M. Technological Aspects of Highly Selective Synthesis of Allyloxyalcohols—New, Greener, Productive Methods. Catalysts 2021, 11, 1559. https://doi.org/10.3390/catal11121559
Urbala M. Technological Aspects of Highly Selective Synthesis of Allyloxyalcohols—New, Greener, Productive Methods. Catalysts. 2021; 11(12):1559. https://doi.org/10.3390/catal11121559
Chicago/Turabian StyleUrbala, Magdalena. 2021. "Technological Aspects of Highly Selective Synthesis of Allyloxyalcohols—New, Greener, Productive Methods" Catalysts 11, no. 12: 1559. https://doi.org/10.3390/catal11121559