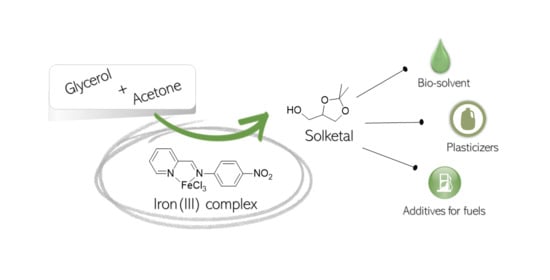
Kinetic Modeling of Solketal Synthesis from Glycerol and Acetone Catalyzed by an Iron(III) Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. Kinetic Experiments of Glycerol Ketalization with Acetone Promoted by FeCl3(1-NO2)
2.2. Kinetic Modeling
3. Materials and Method
3.1. Materials
3.2. Catalyst Preparation
3.3. Reactor Setup and Procedure
3.4. Analytical Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | Acetone |
| BP | By-product |
| CI | Confidence interval |
| EXP | Experimental data |
| Gly | Glycerol |
| SIM | Simulated data |
| List of Symbols | Explanation |
| ci | Concentration of component i [mol/m3] |
| Ea | Activation energy of reaction [kJ/mol] |
| K | Equilibrium constant [-] |
| Kref | Reference equilibrium constant [-] |
| k | Kinetic constant [(m3/mol) s−1] |
| kref | Reference kinetic constant [(m3/mol) s−1] |
| r | Rate of the reaction [mol/(m3 s)] |
| R | Ideal gas constant [kJ/(K mol)] |
| t | Time [s] |
| T | Temperature [K] |
| Tref | Reference temperature (303 K) [K] |
| v | Stirring speed [rpm] |
| XGly | Glycerol conversion degree [-] |
| Greek Letters | |
| ΔrH | Reaction enthalpy [kJ/mol] |
| η | Catalyst form [mol] |
| νi | Stoichiometric coefficient of component i [-] |
| ρCAT | Catalyst bulk density [kg/m3] |
| ɸsolketal | Selectivity towards solketal [-] |
References
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts. Green Chem. 2012, 14, 1611–1619. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C.C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Ilgen, O.; Yerlikaya, S.; Akyurek, F.O. Synthesis of Solketal from Glycerol and Acetone over Amberlyst-46 to Produce an Oxygenated Fuel Additive. Period. Polytech. Chem. Eng. 2016, 61, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.J.A.; da Silva, C.X.A.; Rosenbach, N.; Costa, J.; Da Silva, F. Glycerin Derivatives as Fuel Additives: The Addition of Glycerol/Acetone Ketal (Solketal) in Gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Suriyaprapadilok, N.; Kitiyanan, B. Synthesis of Solketal from Glycerol and Its Reaction with Benzyl Alcohol. Energy Procedia 2011, 9, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Gui, Z.; Zahrtmann, N.; Saravanamurugan, S.; Reyero, I.; Qi, Z.; Bañares, M.A.; Riisager, A.; García-Suárez, E.J. Brønsted Acid Ionic Liquids (BAILs) as Efficient and Recyclable Catalysts in the Conversion of Glycerol to Solketal at Room Temperature. Chem. 2016, 1, 5869–5873. [Google Scholar] [CrossRef] [Green Version]
- da Silva, G.P.; De Lima, C.J.B.; Contiero, J. Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneuoniae GLC29. Catal. Today 2015, 257, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Royon, D.; Locatelli, S.; Gonzo, E. Ketalization of glycerol to solketal in supercritical acetone. J. Supercrit. Fluids 2011, 58, 88–92. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. (Max) Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry and resource efficiency: Towards a green economy. Green Chem. 2016, 18, 3180–3183. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Turco, R.; Tesser, R.; Cucciolito, M.E.; Fagnano, M.; Ottaiano, L.; Mallardo, S.; Malinconico, M.; Santagata, G.; Di Serio, M. Cynara cardunculus Biomass Recovery: An Eco-Sustainable, Nonedible Resource of Vegetable Oil for the Production of Poly(lactic acid) Bioplasticizers. ACS Sustain. Chem. Eng. 2019, 7, 4069–4077. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; De Santis, A.; Di Serio, M.; Esposito, R.; Melchiorre, M.; Nugnes, F.; Paduano, L.; Ruffo, F. A Sustainable Process for the Production of Varnishes Based on Pelargonic Acid Esters. J. Am. Oil Chem. Soc. 2019, 96, 443–461. [Google Scholar] [CrossRef]
- Russo, V.; Taddeo, F.; Cogliano, T.; Vitiello, R.; Esposito, R.; Tesser, R.; Salmi, T.; Di Serio, M. Investigation of the intrinsic reaction kinetics and the mass transfer phenomena of nonanoic acid esterification with 2-ethylhexanol promoted by Sulfuric acid or Amberlite IR120. Chem. Eng. J. 2020, 127236. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; García-Ochoa, F. Kinetic modelling of the solventless synthesis of solketal with a sulphonic ion exchange resin. Chem. Eng. J. 2015, 269, 194–202. [Google Scholar] [CrossRef]
- Alsawalha, M. Catalytic Activity and Kinetic Modeling of Various Modules HZMS-5 and Treated MCM-41 Catalysts, for the Liquid-Phase Ketalization of Glycerol With Acetone. Front. Chem. 2019, 7, 799. [Google Scholar] [CrossRef]
- Esposito, R.; Raucci, U.; Cucciolito, M.E.; Di Guida, R.; Scamardella, C.; Rega, N.; Ruffo, F. Iron(III) Complexes for Highly Efficient and Sustainable Ketalization of Glycerol: A Combined Experimental and Theoretical Study. ACS Omega 2019, 4, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.S.; Sudarsanam, P.; Mallesham, B.; Raju, G.; Reddy, B.M. Acetalisation of glycerol with acetone over zirconia and promoted zirconia catalysts under mild reaction conditions. J. Ind. Eng. Chem. 2011, 17, 377–381. [Google Scholar] [CrossRef]
- Clarkson, J.S.; Walker, A.J.; Wood, M.A. Continuous Reactor Technology for Ketal Formation: An Improved Synthesis of Solketal. Org. Process. Res. Dev. 2001, 5, 630–635. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Rossa, V.; Pessanha, Y.D.S.P.; Díaz, G.C.; Câmara, L.D.T.; Pergher, S.B.C.; Aranda, D.A.G. Reaction Kinetic Study of Solketal Production from Glycerol Ketalization with Acetone. Ind. Eng. Chem. Res. 2017, 56, 479–488. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Nekhaev, A.I.; Ramazanov, D.N.; Arinicheva, Y.; Dzyubenko, A.A.; Khadzhiev, S.N.; Maximov, A.L. Preparation of high-octane oxygenate fuel components from plant-derived polyols. Pet. Chem. 2011, 51, 61–69. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.; Ramos, A.; Vital, J.; Castanheiro, J. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B: Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Esposito, R.; Cucciolito, M.E.; D’Amora, A.; Di Guida, R.; Montagnaro, F.; Ruffo, F. Highly efficient iron(III) molecular catalysts for solketal production. Fuel Process. Technol. 2017, 167, 670–673. [Google Scholar] [CrossRef]
- Melchiorre, M.; Amendola, R.; Benessere, V.; Cucciolito, M.E.; Ruffo, F.; Esposito, R. Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron(III) dimer complex. Mol. Catal. 2020, 483, 110777. [Google Scholar] [CrossRef]
- Esposito, R.; Melchiorre, M.; Annunziata, A.; Cucciolito, M.E.; Ruffo, F. Emerging catalysis in biomass valorisation: Simple Zn(II) catalysts for fatty acids esterification and transesterification. ChemCatChem 2020, 12, 5858. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Dal Poggetto, G.; Di Serio, M.; Granados, M.L.; Ruffo, F.; Vitagliano, A.; Vitiello, R. Strategies for immobilizing homogeneous zinc catalysts in biodiesel production. Catal. Commun. 2014, 56, 81–85. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Esposito, R.; Lega, M.; Turco, R.; Ruffo, F.; Di Serio, M. A novel and robust homogeneous supported catalyst for biodiesel production. Fuel 2016, 171, 1–4. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; De Santis, A.; Di Serio, M.; Esposito, R.; Ruffo, F.; Turco, R. Sustainable Process for Production of Azelaic Acid Through Oxidative Cleavage of Oleic Acid. J. Am. Oil Chem. Soc. 2015, 92, 1701–1707. [Google Scholar] [CrossRef]
- Melchiorre, M.; Benessere, V.; Cucciolito, M.E.; Melchiorre, C.; Ruffo, F.; Esposito, R. Direct and Solvent-Free Oxidative Cleavage of Double Bonds in High-Oleic Vegetable Oils. ChemistrySelect 2020, 5, 1396–1400. [Google Scholar] [CrossRef]
- Annunziata, A.; Esposito, R.; Gatto, G.; Cucciolito, M.E.; Tuzi, A.; Macchioni, A.; Ruffo, F. Iron(III) Complexes with Cross-Bridged Cyclams: Synthesis and Use in Alcohol and Water Oxidation Catalysis. Eur. J. Inorg. Chem. 2018, 2018, 3304–3311. [Google Scholar] [CrossRef]
- Menezes, F.D.L.; Guimaraes, M.D.O.; da Silva, M.J. Highly Selective SnCl2-Catalyzed Solketal Synthesis at Room Temperature. Ind. Eng. Chem. Res. 2013, 52, 16709–16713. [Google Scholar] [CrossRef]
- Du, H.; Deng, F.; Kommalapati, R.R.; Amarasekara, A.S. Iron based catalysts in biomass processing. Renew. Sustain. Energy Rev. 2020, 134, 110292. [Google Scholar] [CrossRef]
- Moreira, M.; Faria, R.P.; Ribeiro, A.M.; Rodrigues, A.E. Solketal Production from Glycerol Ketalization with Acetone: Catalyst Selection and Thermodynamic and Kinetic Reaction Study. Ind. Eng. Chem. Res. 2019, 58, 17746–17759. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Esteban, J.; García-Ochoa, F.; Ladero, M. Solventless synthesis of solketal with commercially available sulfonic acid based ion exchange resins and their catalytic performance. Green Process. Synth. 2017, 6, 79–89. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J.; Behr, A.; Ladero, M.; Garcia-Ochoa, F. Liquid–Liquid Equilibria for the System Acetone + Solketal + Glycerol at (303.2, 313.2, and 323.2) K. J. Chem. Eng. Data 2014, 59, 2850–2855. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- da Silva, M.J.; Rodrigues, A.A.; Pinheiro, P.F. Solketal synthesis from glycerol and acetone in the presence of metal salts: A Lewis or Brønsted acid catalyzed reaction? Fuel 2020, 276, 118164. [Google Scholar] [CrossRef]









| Run | v [rpm] | T [K] | Ac/Gly [mol/mol] | ρCAT [kg/m3] |
|---|---|---|---|---|
| B | 400 | 323 | 4:1 | - |
| C1 | 600 | 323 | 4:1 | 1.39 |
| C2 | 400 | 323 | 4:1 | 1.39 |
| C3 | 400 | 323 | 4:1 | 2.62 |
| C4 | 400 | 323 | 4:1 | 0.67 |
| C5 | 400 | 323 | 4:1 | 0.33 |
| C6 | 400 | 323 | 4:1 | 0.08 |
| C7 | 400 | 323 | 4:1 | 0.04 |
| C8 | 400 | 323 | 4:1 | 0.02 |
| C9 | 400 | 313 | 4:1 | 0.02 |
| C10 | 400 | 303 | 4:1 | 0.02 |
| C11 | 400 | 303 | 4:1 | 0.04 |
| C12 | 400 | 303 | 4:1 | 0.08 |
| C13 | 400 | 303 | 4:1 | 0.25 |
| Value | 95% CI | Units | |
|---|---|---|---|
| ΔrH1 | 30 | 1 | kJ/mol |
| ΔrH2 | 16.4 | 0.5 | kJ/mol |
| Ea1 | 13.0 | 0.5 | kJ/mol |
| Ea2 | 64 | 2 | kJ/mol |
| Ea3 | 63 | 3 | kJ/mol |
| Kref,1 | 0.55 | 0.05 | - |
| Kref,2 | 0.10 | 0.02 | - |
| Kref,3 | 8.1 × 10−3 | 0.1 × 10−3 | - |
| kref,1 | 620 | 10 | (m3/mol) s−1 |
| kref,2 | 14.0 × 107 | 0.2 × 107 | (m3/mol) s−1 |
| kref,3 | 42.0 × 105 | 0.5 × 105 | (m3/mol) s−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taddeo, F.; Esposito, R.; Russo, V.; Di Serio, M. Kinetic Modeling of Solketal Synthesis from Glycerol and Acetone Catalyzed by an Iron(III) Complex. Catalysts 2021, 11, 83. https://doi.org/10.3390/catal11010083
Taddeo F, Esposito R, Russo V, Di Serio M. Kinetic Modeling of Solketal Synthesis from Glycerol and Acetone Catalyzed by an Iron(III) Complex. Catalysts. 2021; 11(1):83. https://doi.org/10.3390/catal11010083
Chicago/Turabian StyleTaddeo, Francesco, Roberto Esposito, Vincenzo Russo, and Martino Di Serio. 2021. "Kinetic Modeling of Solketal Synthesis from Glycerol and Acetone Catalyzed by an Iron(III) Complex" Catalysts 11, no. 1: 83. https://doi.org/10.3390/catal11010083