Co-Promoted Ni Nanocatalysts Derived from NiCoAl-LDHs for Low Temperature CO2 Methanation
Abstract
:1. Introduction
2. Results
2.1. Structural and Morphology Characterization of Samples
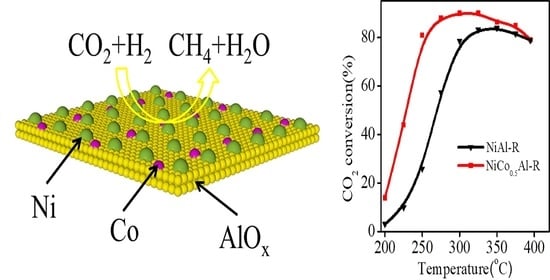
2.2. Catalytic Activity Tests
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Huang, H.; Zhang, Z.; Wang, C.; Yang, Y.; Li, Q.; Xu, D. Lead-free perovskite Cs2AgBiBr6@g-C3N4 Z-scheme system for improving CH4 production in photocatalytic CO2 reduction. Appl. Catal. B 2021, 282, 119570. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Ravilla, D.; Kaleem, W.; Jadhawar, P.; Sharma, T. Impact of low salinity water injection on CO2 storage and oil Recovery for improved CO2 utilization. Chem. Eng. Sci. 2021, 229, 116127. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Reina, T.; Efstathiou, A.; Wang, Q. Aqueous miscible organic LDH derived Ni-based catalysts for efficient CO2 methanation. Catalysts 2020, 10, 1168. [Google Scholar] [CrossRef]
- Spring, A.; Ilyina, T.; Marotzke, J. Inherent uncertainty disguises attribution of reduced atmospheric CO2 growth to CO2 emission reductions for up to a decade. Environ. Res. Lett. 2020, 15, 114058. [Google Scholar] [CrossRef]
- Ishida, H.; Machan, C.; Robert, M.; Iwasawa, N. Editorial: Molecular catalysts for CO2 fixation/reduction. Front. Chem. 2020, 8, 59. [Google Scholar] [CrossRef]
- Rafiee, A. Modelling and optimization of methanol synthesis from hydrogen and CO2. J. Environ. Chem. Eng. 2020, 8, 104314. [Google Scholar] [CrossRef]
- Duyar, M.S.; Tsai, C.; Snider, J.L.; Singh, J.A.; Gallo, A.; Yoo, J.S.; Medford, A.J.; Abild-Pedersen, F.; Studt, F.; Kibsgaard, J.; et al. A highly active molybdenum phosphide catalyst for methanol synthesis from CO and CO2. Angew. Chem. Int. Ed. Engl. 2018, 57, 15045–15050. [Google Scholar] [CrossRef]
- Burger, T.; Donaubauer, P.; Hinrichsen, O. On the kinetics of the co-methanation of CO and CO2 on a co-precipitated Ni-Al catalyst. Appl. Catal. B 2021, 282, 119408. [Google Scholar] [CrossRef]
- Lehtinen, T.; Virtanen, H.; Santala, S.; Santala, V. Production of alkanes from CO2 by engineered bacteria. Biotechnol. Biofuels 2018, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Lv, P.; Yuan, H.; Yang, L.; Wang, Z.; Yuan, Z.; Chen, Y. Insight into forced hydrogen re-arrangement and altered reaction pathways in a protocol for CO2 catalytic processing of oleic acid into C8–C15 alkanes. Green Chem. 2017, 19, 4157–4168. [Google Scholar] [CrossRef]
- Ghaib, K.; Nitz, K.; Ben-Fares, F.Z. Chemical methanation of CO2: A review. ChemBioEng Rev. 2016, 3, 266–275. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mohammadi, M.; Sedighi, M. Sustainable production of light olefins from greenhouse gas CO2 over SAPO-34 supported modified cerium oxide. Microporous Mesoporous Mater. 2020, 297, 110029. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, W.; Zi, L.; Xu, X.; Liu, Q.; Zhong, Q.; Xu, Y. Promotional effect of ZrO2 on supported FeCoK catalysts for ethylene synthesis from catalytic CO2 hydrogenation. Int. J. Hydrogen Energy 2020, 45, 15254–15262. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Fei, Z.; Liu, Q.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Qiao, X. Enhanced light olefin production in chloromethane coupling over Mg/Ca modified durable HZSM-5 catalyst. Ind. Eng. Chem. Res. 2019, 58, 5131–5139. [Google Scholar] [CrossRef]
- Siudyga, T.; Kapkowski, M.; Janas, D.; Wasiak, T.; Sitko, R.; Zubko, M.; Szade, J.; Balin, K.; Klimontko, J.; Lach, D.; et al. Nano-Ru supported on Ni nanowires for low-temperature carbon dioxide methanation. Catalysts 2020, 10, 513. [Google Scholar] [CrossRef]
- Siudyga, T.; Kapkowski, M.; Bartczak, P.; Zubko, M.; Szade, J.; Balin, K.; Antoniotti, S.; Polanski, J. Ultra-low temperature carbon (di)oxide hydrogenation catalyzed by hybrid ruthenium–nickel nanocatalysts: Towards sustainable methane production. Green Chem. 2020, 22, 5143–5150. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Q.; Gu, J. Effective synthesis of zeolite encapsulated Ni nanoparticles with excellent catalytic performance for hydrogenation of CO2 to CH4. Dalton Trans. 2020, 49, 14771–14775. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.; Li, Z.; Song, Y.; Zhang, J.; Wang, J.; He, X.; Wang, C.; Lin, W. Highly dispersed Ni catalyst on metal-organic framework-derived porous hydrous zirconia for CO2 methanation. ACS Appl. Mater. Interfaces 2020, 12, 17436–17442. [Google Scholar] [CrossRef]
- Kikkawa, S.; Teramura, K.; Asakura, H.; Hosokawa, S.; Tanaka, T. Ni–Pt alloy nanoparticles with isolated Pt atoms and their cooperative neighboring Ni atoms for selective hydrogenation of CO2 toward CH4 evolution: In situ and transient fourier transform infrared studies. ACS Appl. Nano Mater. 2020, 3, 9633–9644. [Google Scholar] [CrossRef]
- Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 methanation of biogas over 20 wt% Ni-Mg-Al catalyst: On the effect of N2, CH4, and O2 on CO2 conversion rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Lu, B.; Zhuang, J.; Du, J.; Gu, F.; Xu, G.; Zhong, Z.; Liu, Q.; Su, F. Highly dispersed Ni nanocatalysts derived from NiMnAl-hydrotalcites as high-performing catalyst for low-temperature syngas methanation. Catalysts 2019, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Mutz, B.; Carvalho, H.W.P.; Mangold, S.; Kleist, W.; Grunwaldt, J.D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B 2020, 264, 118494. [Google Scholar] [CrossRef]
- Wang, W.; Duong-Viet, C.; Ba, H.; Baaziz, W.; Tuci, G.; Caporali, S.; Nguyen-Dinh, L.; Ersen, O.; Giambastiani, G.; Pham-Huu, C. Nickel nanoparticles decorated nitrogen-doped carbon nanotubes (Ni/N-CNT): A robust catalyst for the efficient and selective CO2 methanation. ACS Appl. Energy Mater. 2018, 2, 1111–1120. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, B.; Liu, Y.; Li, Y. Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: Current status and perspective. Catal. Today 2015, 256, 29–40. [Google Scholar] [CrossRef]
- Wolf, M.; Wong, L.H.; Schüler, C.; Hinrichsen, O. CO2 methanation on transition-metal-promoted Ni-Al catalysts: Sulfur poisoning and the role of CO2 adsorption capacity for catalyst activity. J. CO2 Util. 2020, 36, 276–287. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, G.A.; González-Velasco, J.R. Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl. Catal. B 2018, 238, 393–403. [Google Scholar] [CrossRef]
- Ahmad, W.; Younis, M.N.; Shawabkeh, R.; Ahmed, S. Synthesis of lanthanide series (La, Ce, Pr, Eu & Gd) promoted Ni/γ-Al2O3 catalysts for methanation of CO2 at low temperature under atmospheric pressure. Catal. Commun. 2017, 100, 121–126. [Google Scholar]
- Xu, L.; Wang, F.; Chen, M.; Zhang, J.; Yuan, K.; Wang, L.; Wu, K.; Xu, G.; Chen, W. CO2 methanation over a Ni based ordered mesoporous catalyst for the production of synthetic natural gas. RSC Adv. 2016, 6, 28489–28499. [Google Scholar] [CrossRef]
- Abate, S.; Mebrahtu, C.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Catalytic Performance of γ-Al2O3–ZrO2–TiO2–CeO2 composite oxide supported Ni-based catalysts for CO2 methanation. Ind. Eng. Chem. Res. 2016, 55, 4451–4460. [Google Scholar] [CrossRef]
- Huang, X.; Wang, P.; Zhang, Z.; Zhang, S.; Du, X.; Bi, Q.; Huang, F. Efficient conversion of CO2 to methane using thin-layer SiOx matrix anchored nickel catalysts. New J. Chem. 2019, 43, 13217–13224. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Q.; Yoneyama, Y.; Tsubaki, N. Nanoparticle modified Ni-based bimodal pore catalysts for enhanced CO2 methanation. RSC Adv. 2014, 4, 64617–64624. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.S.; Antonucci, P.L. The role of gadolinia doped ceria support on the promotion of CO2 methanation over Ni and NiFe catalysts. Int. J. Hydrogen Energy 2017, 42, 26828–26842. [Google Scholar] [CrossRef]
- Huang, T.J.; Yu, T.C. Effect of steam and carbon dioxide pretreatments on methane decomposition and carbon gasification over doped-ceria supported nickel catalyst. Catal. Lett. 2005, 102, 175–181. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, B.; Sun, P. Highly stable Ni-based catalysts derived from LDHs supported on zeolite for CO2 methanation. Int. J. Hydrogen Energy 2020, 45, 16183–16192. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Su, Y.; Shao, D.; Zeng, S.; Wang, W. Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-layered double hydroxide nanosheets. J. Mater. Chem. A 2018, 6, 8366–8373. [Google Scholar] [CrossRef]
- Chen, G.; Gao, R.; Zhao, Y.; Li, Z.; Waterhouse, G.I.N.; Shi, R.; Zhao, J.; Zhang, M.; Shang, L.; Sheng, G.; et al. Alumina-supported CoFe alloy catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 hydrogenation to hydrocarbons. Adv. Mater. 2018, 30, 1704663. [Google Scholar] [CrossRef]
- Martins, J.A.; Faria, A.C.; Soria, M.A.; Miguel, C.V.; Rodrigues, A.E.; Madeira, L.M. CO2 methanation over hydrotalcite-derived nickel/ruthenium and supported ruthenium catalysts. Catalysts 2019, 9, 1008. [Google Scholar] [CrossRef] [Green Version]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni-Fe/(Mg, Al)Ox catalysts for CO2 methanation-tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 8, 1016–1027. [Google Scholar] [CrossRef]
- Aider, N.; Touahra, F.; Bali, F.; Djebarri, B.; Lerari, D.; Bachari, K.; Halliche, D. Improvement of catalytic stability and carbon resistance in the process of CO2 reforming of methane by CoAl and CoFe hydrotalcite-derived catalysts. Int. J. Hydrog. Energy 2018, 43, 8256–8266. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baranc, R.; Dębeka, R.; Motaka, M.; Gálvezb, M.E.; Grzybeka, T.; Costab, P.D.; Glatzelc, P. Examination of the influence of La promotion on Ni state in hydrotalcite derived catalysts under CO2 methanation reaction conditions operando X-ray absorption and emission spectroscopy investigation. Appl. Catal. B 2018, 232, 409–419. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Tang, G.; Gong, D.; Liu, H.; Wang, L. Highly loaded mesoporous Ni–La2O3 catalyst prepared by colloidal solution combustion method for CO2 methanation. Catalysts 2019, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Yashima, T. Supported Co catalysts for methane reforming with CO2. React. Kinet. Catal. Lett. 2004, 81, 153–159. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: Relationship between Co3O4-CeO2 interaction and catalytic activity. Appl. Catal. B 2006, 66, 217–227. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.; Pereira, M.F.R.; Orfao, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interface Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, L.; Zheng, Y.; Zhang, S.; Liu, Q.; Gao, G.; Dong, D.; Wang, Y.; Xu, L.; Hu, X. Methanation of CO2 over nickel catalysts: Impacts of acidic/basic sites on formation of the reaction intermediates. Fuel 2020, 262, 116521. [Google Scholar] [CrossRef]
- Liu, J.; Cao, A.; Si, J.; Zhang, L.; Hao, Q.; Liu, Y. Nanoparticles of Ni-Co alloy derived from layered double hydroxides and their catalytic performance for CO methanation. Nano 2016, 11, 1650118. [Google Scholar] [CrossRef]
- Choi, H.; Oh, S.; Tran, S.B.T.; Park, J.Y. Size-controlled model Ni catalysts on Ga2O3 for CO2 hydrogenation to methanol. J. Catal. 2019, 376, 68–76. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt-palladium nanocatalysts for CO oxidation. Nat. Catal. 2018, 2, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.S.; Kim, J.; Song, H.C.; Kim, D.; Jeong, B.; Lee, J.; Shin, J.W.; Ryoo, R.; Park, J.Y. Catalytic synergy on PtNi bimetal catalysts driven by interfacial intermediate structures. ACS Catal. 2020, 10, 10459–10467. [Google Scholar] [CrossRef]
- Fan, Q.; Li, X.; Yang, Z.; Han, J.; Xu, S.; Zhang, F. Double-confined nickel nanocatalyst derived from layered double hydroxide precursor: Atomic scale insight into microstructure evolution. Chem. Mater. 2016, 28, 6296–6304. [Google Scholar] [CrossRef]











| Samples | a SBET (m2·g−1) | b Vp (m3·g−1) | c Pore Diameter (nm) | d Basic Sites Percentage (%) | |
|---|---|---|---|---|---|
| 65 °C–195 °C | 205 °C–450 °C | ||||
| NiAl-R | 116 | 0.2 | 5 | 32 | 68 |
| NiCo0.25Al-R | 118 | 0.6 | 16 | 20 | 80 |
| NiCo0.5Al-R | 117 | 0.5 | 16 | 14 | 86 |
| NiCo1Al-R | 114 | 0.4 | 15 | 23 | 77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Lu, B.; Sun, P. Co-Promoted Ni Nanocatalysts Derived from NiCoAl-LDHs for Low Temperature CO2 Methanation. Catalysts 2021, 11, 121. https://doi.org/10.3390/catal11010121
Zhang F, Lu B, Sun P. Co-Promoted Ni Nanocatalysts Derived from NiCoAl-LDHs for Low Temperature CO2 Methanation. Catalysts. 2021; 11(1):121. https://doi.org/10.3390/catal11010121
Chicago/Turabian StyleZhang, Fanying, Bin Lu, and Peiqin Sun. 2021. "Co-Promoted Ni Nanocatalysts Derived from NiCoAl-LDHs for Low Temperature CO2 Methanation" Catalysts 11, no. 1: 121. https://doi.org/10.3390/catal11010121