Synthesis, Characterization, and Anti-Algal Activity of Molybdenum-Doped Metal Oxides
Abstract
:1. Introduction
2. Results
2.1. Synthesis, Morphological and Microstructural Analysis of MoZnO
2.2. Structural Analysis of MoZnO
2.3. Anti-Algal Assay
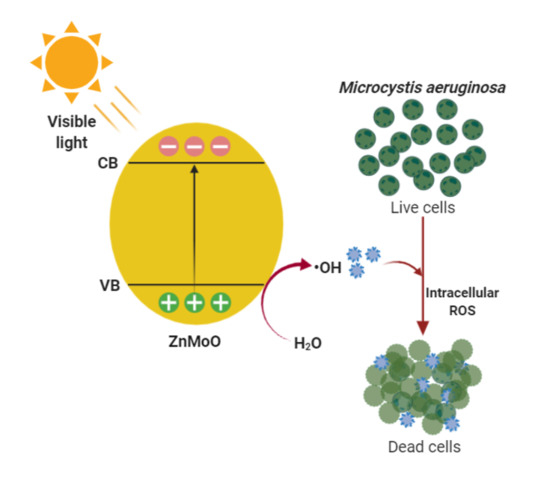
2.4. Mechanisms of Algae Growth Inhibition
3. Discussion
4. Materials and Methods
4.1. Preparation of MoZnO
4.2. Characterization
4.2.1. Morphological and Microstructural Analysis
4.2.2. Structural Analysis
XRD Analysis
FT-IR Analysis
4.3. Algae Growth Inhibition
4.3.1. Algae Culture
4.3.2. Anti-Algal Activity
4.3.3. Effect of Metal Salts, Metal Oxides and Their Combinations
4.4. Experiments on Anti-Algal Mechanisms
4.4.1. Hydroxyl Radical (·OH) Assay
4.4.2. ROS Assay
4.4.3. Lipid Peroxidation Assay
4.4.4. Effect of Agglomeration
Optical Microscope Analysis
SEM Analysis
4.4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.; Anariba, F.; Tan, J.C.; Li, X.; Wu, P. A review of physiochemical and photocatalytic properties of metal oxides against Escherichia coli. J. Photochem. Photobiol. A Chem. 2018, 360, 306–315. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, L.; Yan, H.; Wang, X.; Liu, X.; Zhang, X.; Huang, X.; Hang, R.; Wang, Y.; Yao, X.; et al. Bovine serum albumin assisted synthesis of Ag/Ag2O/ZnO photocatalyst with enhanced photocatalytic activity under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 131–140. [Google Scholar] [CrossRef]
- Shu, Z.; Jiao, X.; Chen, D. Synthesis and photocatalytic properties of flower-like zirconia nanostructures. CrystEngComm 2012, 14, 1122–1127. [Google Scholar] [CrossRef]
- Yan, X.; Hu, B.; Lu, W.; Sun, S.; Shi, W.; Wang, X. Enhanced photocatalytic activity induced by surface plasmon resonance on Ag-loaded strontium titanate nanoparticles. Micro Nano Lett. 2013, 8, 504–507. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. Influence of molybdenum doping on the structural, optical and electronic properties of WO3 for improved solar water splitting. J. Colloid Interface Sci. 2018, 509, 440–447. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Hossienzadeh, K.; Maleki, A.; Ghanbari, R.; Rezaee, R.; Safari, M.; Shahmoradi, B.; Daraei, H.; Jafari, A.; Yetilmezsoy, K.; et al. Effects of doping zinc oxide nanoparticles with transition metals (Ag, Cu, Mn) on photocatalytic degradation of Direct Blue 15 dye under UV and visible light irradiation. J. Environ. Health Sci. Eng. 2019, 17, 479–492. [Google Scholar] [CrossRef]
- Gouda, M. Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. J. Ind. Text. 2011, 41, 222–240. [Google Scholar] [CrossRef]
- Ghasempour, F.; Azimirad, R.; Amini, A.; Akhavan, O. Visible light photoinactivation of bacteria by tungsten oxide nanostructures formed on a tungsten foil. Appl. Surf. Sci. 2015, 338, 55–60. [Google Scholar] [CrossRef]
- Autefage, H.; Allen, F.; Tang, H.; Kallepitis, C.; Gentleman, E.; Reznikov, N.; Nitiputri, K.; Nommeots-Nomm, A.; O’Donnell, M.; Lange, C.; et al. Multiscale analyses reveal native-like lamellar bone repair and near perfect bone-contact with porous strontium-loaded bioactive glass. Biomaterials 2019, 209, 152–162. [Google Scholar] [CrossRef]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Abutalib, M.; Yahia, I. Novel and facile microwave-assisted synthesis of Mo-doped hydroxyapatite nanorods: Characterization, gamma absorption coefficient, and bioactivity. Mater. Sci. Eng. C 2017, 78, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Vayaa, D.; Sharmab, V.K. Study of synthesis and photocatalytic activities of Mo doped ZnO. J. Chem. Pharm. 2011, 3, 234–244. [Google Scholar]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Nasir, Z.; Shakir, M.; Wahab, R.; Shoeb, M.; Alam, P.; Khan, R.; Mobin, M.; Lutfullah, L. Co-precipitation synthesis and characterization of Co doped SnO2 NPs, HSA interaction via various spectroscopic techniques and their antimicrobial and photocatalytic activities. Int. J. Biol. Macromol. 2017, 94, 554–565. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel one-step synthesis of sulfur doped-TiO2 by flame spray pyrolysis for visible light photocatalytic degradation of acetaldehyde. Chem. Eng. J. 2018, 339, 249–258. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.; Abdala, A. Solvent-free synthesis of ZnO-graphene nanocomposite with superior photocatalytic activity. Appl. Surf. Sci. 2019, 465, 1107–1113. [Google Scholar] [CrossRef]
- Ahadi, S.; Moalej, N.S.; Sheibani, S. Characteristics and photocatalytic behavior of Fe and Cu doped TiO2 prepared by combined sol-gel and mechanical alloying. Solid State Sci. 2019, 96, 105975. [Google Scholar] [CrossRef]
- Shafei, A.; Salarpour, M.E.; Sheibani, S. Effect of intermediate ball milling on the synthesis of Cu-doped TiO2 nano-photocatalyst by sol–gel method. J. Sol-Gel Sci. Technol. 2019, 92, 173–185. [Google Scholar] [CrossRef]
- Oukarroum, A.; Halimi, I.; Siaj, M. Cellular Responses of Chlorococcum Sp. Algae Exposed to Zinc Oxide Nanoparticles by Using Flow Cytometry. Water Air Soil Pollut. 2018, 230, 1–7. [Google Scholar] [CrossRef]
- Du, J.; Guo, R.; Li, K.; Ma, B.; Chen, Y.; Lv, Y. Contributions of Zn Ions to ZnO Nanoparticle Toxicity on Microcystis aeruginosa During Chronic Exposure. Bull. Environ. Contam. Toxicol. 2019, 103, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Halbus, A.; Horozov, T.S.; Paunov, V.N. Surface-Modified Zinc Oxide Nanoparticles for Antialgal and Antiyeast Applications. ACS Appl. Nano Mater. 2020, 3, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Sun, L.; Jiang, Y.; Liu, S.; Chen, M.; Chen, M.; Ding, Y.; Liu, Q. A facile strategy for the preparation of ZnS nanoparticles deposited on montmorillonite and their higher catalytic activity for rapidly colorimetric detection of H2O2. Mater. Sci. Eng. C 2016, 67, 188–194. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Zheng, X.; Zhao, W.; Wu, M.; Wang, Z. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat. Toxicol. 2015, 158, 1–13. [Google Scholar] [CrossRef]
- Suman, T.; Rajasree, S.R.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.; Macdonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.A.; Soares, E.V.; Soares, E.V. Chronic exposure of the freshwater alga Pseudokirchneriella subcapitata to five oxide nanoparticles: Hazard assessment and cytotoxicity mechanisms. Aquat. Toxicol. 2019, 214, 105265. [Google Scholar] [CrossRef] [Green Version]
- Joonas, E.; Aruoja, V.; Olli, K.; Kahru, A. Environmental safety data on CuO and TiO2 nanoparticles for multiple algal species in natural water: Filling the data gaps for risk assessment. Sci. Total Environ. 2019, 647, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, G.; Bae, H.; Kim, E.; Moon, B.; Cheon, D.; Tarte, N.H. An External Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye. Catalysts 2019, 9, 642. [Google Scholar] [CrossRef] [Green Version]
- Xiu, X.; Pang, Z.; Lv, M.; Dai, Y.; Ye, L.; Han, S. Transparent conducting molybdenum-doped zinc oxide films deposited by RF magnetron sputtering. Appl. Surf. Sci. 2007, 253, 3345–3348. [Google Scholar] [CrossRef]
- Shatnawi, M.; Alsmadi, A.; Bsoul, I.; Salameh, B.; Mathai, M.; Alnawashi, G.; Alzoubi, G.M.; Al-Dweri, F.; Bawa’Aneh, M. Influence of Mn doping on the magnetic and optical properties of ZnO nanocrystalline particles. Results Phys. 2016, 6, 1064–1071. [Google Scholar] [CrossRef] [Green Version]
- Rusu, D.; Rusu, G.; Luca, D. Structural Characteristics and Optical Properties of Thermally Oxidized Zinc Films. Acta Phys. Pol. A 2011, 119, 850–856. [Google Scholar] [CrossRef]
- Swapna, R.; Kumar, M. Growth and characterization of molybdenum doped ZnO thin films by spray pyrolysis. J. Phys. Chem. Solids 2013, 74, 418–425. [Google Scholar] [CrossRef]
- Boukhachem, A.; Ouni, B.; Karyaoui, M.; Madani, A.; Chtourou, R.; Amlouk, M. Structural, opto-thermal and electrical properties of ZnO:Mo sprayed thin films. Mater. Sci. Semicond. Process. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Azizi, S.; Bin Ahmad, M.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Nakagaki, S.; Bail, A.; Dos Santos, V.C.; Souza, V.H.R.; Vrubel, H.; Nunes, F.S.; Ramos, L.P. Use of anhydrous sodium molybdate as an efficient heterogeneous catalyst for soybean oil methanolysis. Appl. Catal. A Gen. 2008, 351, 267–274. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Shu, Q.; Yu, J.C.-M.; Cao, F.; Li, X.; Zhou, X. Preparation, characterization and photocatalytic performance of Mo-doped ZnO photocatalysts. Sci. China Ser. B Chem. 2012, 55, 1802–1810. [Google Scholar] [CrossRef]
- Afanasiev, P.; Geantet, C.; Kerridge, D.H. Products of reactions of Mo and Zr compounds in molten nitrate. J. Mater. Chem. 1995, 5, 347. [Google Scholar] [CrossRef]
- Craciun, V.; Singh, R.K. Characteristics of the surface layer of barium strontium titanate thin films deposited by laser ablation. Appl. Phys. Lett. 2000, 76, 1932–1934. [Google Scholar] [CrossRef] [Green Version]
- Phongarthit, K.; Amornpitoksuk, P.; Suwanboon, S. Synthesis, characterization, and photocatalytic properties of ZnO nanoparticles prepared by a precipitation-calcination method using a natural alkaline solution. Mater. Res. Express 2019, 6, 045501. [Google Scholar] [CrossRef]
- Quek, J.-A.; Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. Visible light responsive flower-like ZnO in photocatalytic antibacterial mechanism towards Enterococcus faecalis and Micrococcus luteus. J. Photochem. Photobiol. B Biol. 2018, 187, 66–75. [Google Scholar] [CrossRef]
- Hoque, A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, R.A.; Kondrachova, L.; Hahn, B.P.; Stevenson, K.J. Optical Constants of Electrodeposited Mixed Molybdenum−Tungsten Oxide Films Determined by Variable-Angle Spectroscopic Ellipsometry. J. Phys. Chem. C 2007, 111, 18251–18257. [Google Scholar] [CrossRef]
- Chary, K.V. Structure and catalytic properties of molybdenum oxide catalysts supported on zirconia. J. Catal. 2004, 226, 283–291. [Google Scholar] [CrossRef]
- Nosaka, Y.; Takahashi, S.; Mitani, Y.; Qiu, X.; Miyauchi, M. Reaction mechanism of visible-light responsive Cu(II)-grafted Mo-doped SrTiO3 photocatalyst studied by means of ESR spectroscopy and chemiluminescence photometry. Appl. Catal. B Environ. 2012, 111, 636–640. [Google Scholar] [CrossRef]
- Xie, S.; Chen, K.; Bell, A.T.; Iglesia, E. Structural Characterization of Molybdenum Oxide Supported on Zirconia. J. Phys. Chem. B 2000, 104, 10059–10068. [Google Scholar] [CrossRef] [Green Version]
- Madhavi, V.; Kumar, P.J.; Kondaiah, P.; Hussain, O.M.; Uthanna, S. Effect of molybdenum doping on the electrochromic properties of tungsten oxide thin films by RF magnetron sputtering. Ionics 2014, 20, 1737–1745. [Google Scholar] [CrossRef]
- Yin, H.; Kuwahara, Y.; Mori, K.; Cheng, H.; Wen, M.; Huo, Y.; Yamashita, H. Localized Surface Plasmon Resonances in Plasmonic Molybdenum Tungsten Oxide Hybrid for Visible-Light-Enhanced Catalytic Reaction. J. Phys. Chem. C 2017, 121, 23531–23540. [Google Scholar] [CrossRef]
- Fan, G.; Bao, M.; Zheng, X.; Hong, L.; Zhan, J.; Chen, Z.; Qu, F. Growth inhibition of harmful cyanobacteria by nanocrystalline Cu-MOF-74: Efficiency and its mechanisms. J. Hazard. Mater. 2019, 367, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, J.; Wang, Z.; Cai, W. Preparation of visible light-responsive AgBiO3 bactericide and its control effect on the Microcystis aeruginosa. J. Photochem. Photobiol. B Biol. 2010, 101, 265–270. [Google Scholar] [CrossRef]
- He, X.; Xie, C.; Ma, Y.; Wang, L.; He, X.; Shi, W.; Liu, X.; Liu, Y.; Zhang, Z. Size-dependent toxicity of ThO2 nanoparticles to green algae Chlorella pyrenoidosa. Aquat. Toxicol. 2019, 209, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Y.; Chen, S.; Ge, M.; He, J.; Yang, Z.; Lin, P.; Wu, X. White matter integrity correlates with residual consciousness in patients with severe brain injury. Brain Imaging Behav. 2018, 12, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shao, Y.; Gao, N.; Zhu, S.; Li, L.; Deng, J.; Zhu, M. Removal of Microcystis aeruginosa by potassium ferrate (VI): Impacts on cells integrity, intracellular organic matter release and disinfection by-products formation. Chem. Eng. J. 2014, 251, 304–309. [Google Scholar] [CrossRef]
- Mitran, G.; Neaţu, F.; Pavel, O.D.; Trandafir, M.M.; Florea, M. Behavior of Molybdenum-Vanadium Mixed Oxides in Selective Oxidation and Disproportionation of Toluene. Materials 2019, 12, 748. [Google Scholar] [CrossRef] [Green Version]
- Znaidi, L.; Touam, T.; Vrel, D.; Souded, N.; Ben Yahia, S.; Brinza, O.; Fischer, A.; Boudrioua, A. AZO Thin Films by Sol-Gel Process for Integrated Optics. Coatings 2013, 3, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Chen, X.; Ma, N.; Zhang, Q.; Qu, D.; Li, M. Overvalued allelopathy and overlooked effects of humic acid-like substances on Microcystis aeruginosa and Scenedesmus obliquus competition. Harmful Algae 2018, 78, 18–26. [Google Scholar] [CrossRef]
- Fan, G.; You, Y.; Wang, B.; Wu, S.; Zhang, Z.; Zheng, X.; Bao, M.; Zhan, J. Inactivation of harmful cyanobacteria by Ag/AgCl@ZIF-8 coating under visible light: Efficiency and its mechanisms. Appl. Catal. B Environ. 2019, 256, 123767. [Google Scholar] [CrossRef]
- Metzler, D.M.; Erdem, A.; Huang, C. Influence of Algae Age and Population on the Response to TiO2 Nanoparticles. Int. J. Environ. Res. Public Health 2018, 15, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, E.R.; Inostroza-Blancheteau, C.; Obando, V.; Rubió, L.; Marcos, R. Genotoxicity of copper oxide nanoparticles in Drosophila melanogaster. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 791, 1–11. [Google Scholar] [CrossRef] [PubMed]







| Element | Weight% | Atomic% |
|---|---|---|
| O | 29.1 | 61.6 |
| Cl | 7.1 | 6.8 |
| Zn | 54.5 | 28.2 |
| Mo | 9.1 | 3.2 |
| Material | Dopant Atomic% | Crystallite Size (nm) | Lattice Constant (Å) | c/a | Lattice Strain (ɛ) | |
|---|---|---|---|---|---|---|
| c | a | |||||
| ZnO | 0 | 45 ± 3.5 | 5.2 ± 0.01 | 3.2 ± 0.002 | 1.6 | 0.10 ± 0.03 |
| MoZnO | 3.2 | 4.4 ± 0.61 | 5.2 ± 0.02 | 3.2 ± 0.002 | 1.6 | 4.5 ± 0.5 |
| Material | Dopant% | TC(hkl) | ||||||
|---|---|---|---|---|---|---|---|---|
| (100) | (002) | (101) | (102) | (110) | (103) | (112) | ||
| ZnO | 0 | 0.91 | 0.97 | 0.95 | 1.07 | 1.23 | 1.21 | 0.65 |
| MoZnO | 3.23 | 0.85 | 1.07 | 0.55 | 1.28 | 1.08 | 1.03 | 1.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandanwar, S.; Lee, M.W.; Borkar, S.; Cho, J.H.; Tarte, N.H.; Kim, H.J. Synthesis, Characterization, and Anti-Algal Activity of Molybdenum-Doped Metal Oxides. Catalysts 2020, 10, 805. https://doi.org/10.3390/catal10070805
Nandanwar S, Lee MW, Borkar S, Cho JH, Tarte NH, Kim HJ. Synthesis, Characterization, and Anti-Algal Activity of Molybdenum-Doped Metal Oxides. Catalysts. 2020; 10(7):805. https://doi.org/10.3390/catal10070805
Chicago/Turabian StyleNandanwar, Sondavid, Myung Won Lee, Shweta Borkar, Jeong Hyung Cho, Naresh H. Tarte, and Hak Jun Kim. 2020. "Synthesis, Characterization, and Anti-Algal Activity of Molybdenum-Doped Metal Oxides" Catalysts 10, no. 7: 805. https://doi.org/10.3390/catal10070805