A Simple and Efficient Protocol for Proline-Catalysed Asymmetric Aldol Reaction
Abstract
:1. Introduction
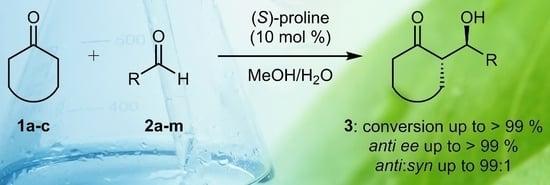
2. Results and Discussion
2.1. Optimization of the Reaction Protocol
2.2. Application of the Protocol to Other Ketones
2.3. Large-Scale Application of the Protocol
2.4. Work-Up Investigations
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures.
3.2.1. General Procedure for the Small-Scale Aldol Condensation Between Aldehydes 2 and Ketones 1
3.2.2. Procedure for the Aldol Condensation Between Benzaldehyde 2d and Cyclohexanone 1a on 10 mmol Scale
3.2.3. Procedure for the Aldol Condensation between Benzaldehyde 2d and Cyclohexanone 1a on 100 mmol Scale (Table 6)
3.2.4. General Procedure for the Study of Reaction Outcome as a Function of Reaction Time (Table 7)
3.2.5. Procedure for the Aldol Condensation between Benzaldehyde 2d (50 mmol) and Cyclohexanone 1a (2 equivalents, 100 mmol)
3.3. Work-Up Procedures
3.3.1. Procedure for Filtration on a Silica-Pad (Method A, Table 8)
3.3.2. Procedure for Aqueous Work-Up Employing NH4Cl (Method B, Table 8)
3.3.3. Procedure for Dilution with EtOAc and Cooling (Method C, Table 8).
3.3.4. Procedure for Dilution with Et2O and Cooling (Method D, Table 8).
3.3.5. Procedure for Dilution with DCM and Cooling (Method E, Table 8)
3.3.6. Procedure for Dilution with n-Hexane and Cooling (Method F, Table 8)
3.4. Products Characterization.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Asymmetric Aminocatalysis-Gold Rush in Organic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 6138–6171. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Pihko, P.M.; Majander, I.; Erkkilä, A. Enamine Catalysis. Top. Curr. Chem. 2009, 291, 145–200. [Google Scholar] [CrossRef]
- Schneider, J.F.; Ladd, C.L.; Bräse, S. Chapter 5: Proline as an Asymmetric Organocatalyst; RSC Publishing: Cambridge, UK, 2015; pp. 79–119. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yasukawa, T.; Yoo, W.-J.; Kitanosono, T.; Kobayashi, S. Catalytic enantioselective aldol reactions. Chem. Soc. Rev. 2018, 47, 4388–4480. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J. Recent Advances in Asymmetric Reactions Catalyzed by Proline and Its Derivatives. Synthesis 2016, 49, 960–972. [Google Scholar] [CrossRef]
- List, B. Proline-catalyzed asymmetric reactions. Tetrahedron 2002, 58, 5573–5590. [Google Scholar] [CrossRef]
- Barbas, C.F. Organocatalysis Lost: Modern Chemistry, Ancient Chemistry, and an Unseen Biosynthetic Apparatus. Angew. Chem. Int. Ed. 2008, 47, 42–47. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; García, J.M. Current progress in the asymmetric aldol addition reaction. Chem. Soc. Rev. 2004, 33, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wennemers, H. Peptides as Asymmetric Catalysts for Aldol Reactions. Chim. Int. J. Chem. 2007, 61, 276–278. [Google Scholar] [CrossRef]
- Notz, W.; Tanaka, F.; Barbas, C.F. Enamine-Based Organocatalysis with Proline and Diamines: The Development of Direct Catalytic Asymmetric Aldol, Mannich, Michael, and Diels−Alder Reactions. Accounts Chem. Res. 2004, 37, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Yang, J.W.; Hoffmann, S.; List, B. Asymmetric Enamine Catalysis. Chem. Rev. 2007, 107, 5471–5569. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P. The Direct Catalytic Asymmetric Cross-Aldol Reaction of Aldehydes. Angew. Chem. Int. Ed. 2003, 42, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P. The Direct Catalytic Asymmetric Aldol Reaction. Eur. J. Org. Chem. 2002, 2002, 1595–1601. [Google Scholar] [CrossRef]
- Guillena, G.; Najera, C.; Ramón, D.J. Enantioselective direct aldol reaction: The blossoming of modern organocatalysis. Tetrahedron Asymmetry 2007, 18, 2249–2293. [Google Scholar] [CrossRef]
- Geary, L.M.; Hultin, P.G. The state of the art in asymmetric induction: The aldol reaction as a case study. Tetrahedron Asymmetry 2009, 20, 131–173. [Google Scholar] [CrossRef]
- Schetter, B.; Mahrwald, R. Modern Aldol Methods for the Total Synthesis of Polyketides. Angew. Chem. Int. Ed. 2006, 45, 7506–7525. [Google Scholar] [CrossRef] [PubMed]
- Mestres, R. A green look at the aldol reaction. Green Chem. 2004, 6, 583. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Houk, K.N.; Martin, H.J.; List, B. Quantum Mechanical Predictions of the Stereoselectivities of Proline-Catalyzed Asymmetric Intermolecular Aldol Reactions. J. Am. Chem. Soc. 2003, 125, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Beck, A.K.; Badine, D.M.; Limbach, M.; Eschenmoser, A.; Treasurywala, A.M.; Hobi, R.; Prikoszovich, W.; Linder, B. Are Oxazolidinones Really Unproductive, Parasitic Species in Proline Catalysis?—Thoughts and Experiments Pointing to an Alternative View. Helvetica Chim. Acta 2007, 90, 425–471. [Google Scholar] [CrossRef]
- Zotova, N.; Franzke, A.; Armstrong, A.; Blackmond, D.G. Clarification of the Role of Water in Proline-Mediated Aldol Reactions. J. Am. Chem. Soc. 2007, 129, 15100–15101. [Google Scholar] [CrossRef] [PubMed]
- Zotova, N.; Broadbelt, L.J.; Armstrong, A.; Blackmond, D.G. Kinetic and mechanistic studies of proline-mediated direct intermolecular aldol reactions. Bioorganic Med. Chem. Lett. 2009, 19, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Sunoj, R.B. Enamine versus Oxazolidinone: What Controls Stereoselectivity in Proline-Catalyzed Asymmetric Aldol Reactions? Angew. Chem. Int. Ed. 2010, 49, 6373–6377. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Ceotto, M.; Benaglia, M. Kinetics versus thermodynamics in the proline catalyzed aldol reaction. Chem. Sci. 2016, 7, 5421–5427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haindl, M.H.; Hioe, J.; Gschwind, R.M. The Proline Enamine Formation Pathway Revisited in Dimethyl Sulfoxide: Rate Constants Determined via NMR. J. Am. Chem. Soc. 2015, 137, 12835–12842. [Google Scholar] [CrossRef] [PubMed]
- Renzi, P.; Hioe, J.; Gschwind, R.M. Enamine/Dienamine and Brønsted Acid Catalysis: Elusive Intermediates, Reaction Mechanisms, and Stereoinduction Modes Based on in Situ NMR Spectroscopy and Computational Studies. Accounts Chem. Res. 2017, 50, 2936–2948. [Google Scholar] [CrossRef] [PubMed]
- Notz, W.; List, B. Catalytic Asymmetric Synthesis ofanti-1,2-Diols. J. Am. Chem. Soc. 2000, 122, 7386–7387. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Castello, C. Proline-Catalyzed Asymmetric Aldol Reactions between Ketones and α-Unsubstituted Aldehydes. Org. Lett. 2001, 3, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C.F. Amino acid catalyzed direct asymmetric aldol reactions: A bioorganic approach to catalytic asymmetric carbon-carbon bond-forming reactions. J. Am. Chem. Soc. 2001, 123, 5260–5267. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Notz, W.; Barbas, C.F. Direct organocatalytic aldol reactions in buffered aqueous media. Chem. Commun. 2002, 24, 3024–3025. [Google Scholar] [CrossRef] [PubMed]
- Pihko, P.M.; Nyberg, A.I.; Usano, A. Proline-Catalyzed Ketone-Aldehyde Aldol Reactions are Accelerated by Water. Synlett 2004, 2004, 1891–1896. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Ding, Q.-P.; Li, Z.; Wang, P.G.; Cheng, J.-P. Proline catalyzed aldol reactions in aqueous micelles: An environmentally friendly reaction system. Tetrahedron Lett. 2003, 44, 3871–3875. [Google Scholar] [CrossRef]
- Darbre, T.; Machuqueiro, M. Zn-Proline catalyzed direct aldol reaction in aqueous media. Chem. Commun. 2003, 1090–1091. [Google Scholar] [CrossRef]
- Hayashi, Y.; Tsuboi, W.; Shoji, M.; Suzuki, N. Application of high pressure, induced by water freezing, to the direct asymmetric aldol reaction. Tetrahedron Lett. 2004, 45, 4353–4356. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Shao, W.-Y.; Zheng, C.-Q.; Huang, Z.-L.; Cai, J.; Deng, Q.-Y. Studies on Direct Stereoselective Aldol Reactions in Aqueous Media. Helvetica Chim. Acta 2004, 87, 1377–1384. [Google Scholar] [CrossRef]
- Trost, B.M.; Brindle, C.S. The direct catalytic asymmetric aldol reaction. Chem. Soc. Rev. 2010, 39, 1600–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-Y.; Chen, Y.; Zheng, C.-Q.; Yin, Y.; Wang, L.; Sun, X.-Q. Highly enantioselective aldol reactions catalyzed by reusable upper rim-functionalized calix[4]arene-based l -proline organocatalyst in aqueous conditions. Tetrahedron 2017, 73, 78–85. [Google Scholar] [CrossRef]
- Benaglia, M. Recoverable and Recyclable Catalysts, 1st ed.; Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Liu, Y.-X.; Sun, Y.-N.; Tan, H.-H.; Tao, J.-C. Asymmetric Aldol Reaction Catalyzed by New Recyclable Polystyrene-supported l-proline in the Presence of Water. Catal. Lett. 2007, 120, 281–287. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Sun, Y.-N.; Tan, H.-H.; Liu, W.; Tao, J.-C. Linear polystyrene anchored l-proline, new recyclable organocatalysts for the aldol reaction in the presence of water. Tetrahedron Asymmetry 2007, 18, 2649–2656. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Marculescu, A.M.; Meo, P.L.; Rielaa, S.; Noto, R. Hydrophobically Directed Aldol Reactions: Polystyrene-SupportedL-Proline as a Recyclable Catalyst for Direct Asymmetric Aldol Reactions in the Presence of Water. Eur. J. Org. Chem. 2007, 2007, 4688–4698. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.; Smart, T.P.; Epps, T.H.; Longbottom, D.A.; O’Reilly, R.K. l-Proline Functionalized Polymers Prepared by RAFT Polymerization and Their Assemblies as Supported Organocatalysts. Macromolecules 2011, 44, 7233–7241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benaglia, M.; Cinquini, M.; Cozzi, F.; Puglisi, A.; Celentano, G. Poly(Ethylene Glycol)-Supported Proline: A Versatile Catalyst for the Enantioselective Aldol and Iminoaldol Reactions. Adv. Synth. Catal. 2002, 344, 533–542. [Google Scholar] [CrossRef]
- Benaglia, M.; Celentano, G.; Cozzi, F. Enantioselective Aldol Condensations Catalyzed by Poly(ethylene glycol)-Supported Proline. Adv. Synth. Catal. 2001, 343, 171–173. [Google Scholar] [CrossRef]
- Calderón, F.; Fernández, R.; Sánchez, F.; Fernández-Mayoralas, A. Asymmetric Aldol Reaction Using Immobilized Proline on Mesoporous Support. Adv. Synth. Catal. 2005, 347, 1395–1403. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M.; Marculescu, A.M.; Noto, R. Polystyrene-supported proline and prolinamide. Versatile heterogeneous organocatalysts both for asymmetric aldol reaction in water and α-selenenylation of aldehydes. Tetrahedron Lett. 2007, 48, 255–259. [Google Scholar] [CrossRef]
- Font, D.; Jimeno, C.; Pericàs, M.A. Polystyrene-Supported Hydroxyproline: An Insoluble, Recyclable Organocatalyst for the Asymmetric Aldol Reaction in Water. Org. Lett. 2006, 8, 4653–4655. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Li, S.; Wang, X.; Ma, J. The high catalytic activity and reusability of the proline functionalized cage-like mesoporous material SBA-16 for the asymmetric aldol reaction proceeding in methanol–water mixed solvent. RSC Adv. 2014, 4, 9292–9299. [Google Scholar] [CrossRef]
- Angeloni, M.; Piermatti, O.; Pizzo, F.; Vaccaro, L. Synthesis of Zirconium Phosphonate SupportedL-Proline as an Effective Organocatalyst for Direct Asymmetric Aldol Addition. Eur. J. Org. Chem. 2014, 2014, 1716–1726. [Google Scholar] [CrossRef]
- Aghahosseini, H.; Ramazani, A.; Ślepokura, K.; Lis, T. The first protection-free synthesis of magnetic bifunctional l-proline as a highly active and versatile artificial enzyme: Synthesis of imidazole derivatives. J. Colloid Interface Sci. 2018, 511, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Doyagüez, E.G.; Calderón, F.; Sánchez, F.; Fernández-Mayoralas, A. Asymmetric Aldol Reaction Catalyzed by a Heterogenized Proline on a Mesoporous Support. The Role of the Nature of Solvents. J. Org. Chem. 2007, 72, 9353–9356. [Google Scholar] [CrossRef] [PubMed]
- Campisciano, V.; Giacalone, F.; Gruttadauria, M. Supported Ionic Liquids: A Versatile and Useful Class of Materials. Chem. Rec. 2017, 17, 918–938. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Tan, R.; Zhao, L.; Yin, D. l-Proline supported on ionic liquid-modified magnetic nanoparticles as a highly efficient and reusable organocatalyst for direct asymmetric aldol reaction in water. Green Chem. 2013, 15, 2422. [Google Scholar] [CrossRef]
- Ferré, M.; Pleixats, R.; Man, M.W.C.; Cattoën, X. Recyclable organocatalysts based on hybrid silicas. Green Chem. 2016, 18, 881–922. [Google Scholar] [CrossRef] [Green Version]
- Aprile, C.; Giacalone, F.; Gruttadauria, M.; Marculescu, A.M.; Noto, R.; Revell, J.D.; Wennemers, H. New ionic liquid-modified silica gels as recyclable materials for l-proline- or H–Pro–Pro–Asp–NH2-catalyzed aldol reaction. Green Chem. 2007, 9, 1328. [Google Scholar] [CrossRef]
- Lombardo, M.; Trombini, C. Ionic Tags in Catalyst Optimization: Beyond Catalyst Recycling. ChemCatChem 2010, 2, 135–145. [Google Scholar] [CrossRef]
- Lombardo, M.; Quintavalla, A.; Chiarucci, M.; Trombini, C. Multiphase Homogeneous Catalysis: Common Procedures and Recent Applications. Synlett 2010, 2010, 1746–1765. [Google Scholar] [CrossRef]
- Lombardo, M.; Pasi, F.; Easwar, S.; Trombini, C. An Improved Protocol for the Direct Asymmetric Aldol Reaction in Ionic Liquids, Catalysed by Onium Ion-Tagged Prolines. Adv. Synth. Catal. 2007, 349, 2061–2065. [Google Scholar] [CrossRef]
- Lombardo, M.; Pasi, F.; Easwar, S.; Trombini, C. Direct Asymmetric Aldol Reaction Catalyzed by an Imidazolium-Tagged trans-4-Hydroxy-l-proline under Aqueous Biphasic Conditions. Synlett 2008, 2471–2474. [Google Scholar] [CrossRef]
- Lombardo, M.; Easwar, S.; Pasi, F.; Trombini, C. The Ion Tag Strategy as a Route to Highly Efficient Organocatalysts for the Direct Asymmetric Aldol Reaction. Adv. Synth. Catal. 2009, 351, 276–282. [Google Scholar] [CrossRef]
- Bhati, M.; Upadhyay, S.; Easwar, S.; Srinivasan, E. Exploring “Through-Bond” Proximity between the Ion Tag and Reaction Site of an Imidazolium-Proline Catalyst for the Direct Asymmetric Aldol Reaction. Eur. J. Org. Chem. 2017, 1788–1793. [Google Scholar] [CrossRef]
- Rosso, C.; Emma, M.G.; Martinelli, A.; Lombardo, M.; Quintavalla, A.; Trombini, C.; Syrgiannis, Z.; Prato, M. A Recyclable Chiral 2-(Triphenylmethyl)pyrrolidine Organocatalyst Anchored to [60]Fullerene. Adv. Synth. Catal. 2019, 361, 2936–2944. [Google Scholar] [CrossRef]
- Bottoni, A.; Lombardo, M.; Miscione, G.P.; Montroni, E.; Quintavalla, A.; Trombini, C. Electrosteric Activation by using Ion-Tagged Prolines: A Combined Experimental and Computational Investigation. ChemCatChem 2013, 5, 2913–2924. [Google Scholar] [CrossRef]
- Montroni, E.; Lombardo, M.; Quintavalla, A.; Trombini, C.; Gruttadauria, M.; Giacalone, F. A Liquid-Liquid Biphasic Homogeneous Organocatalytic Aldol Protocol Based on the Use of a Silica Gel Bound Multilayered Ionic Liquid Phase. ChemCatChem 2012, 4, 1000–1006. [Google Scholar] [CrossRef]
- Montroni, E.; Sanap, S.P.; Trombini, C.; Dhavale, D.D.; Lombardo, M.; Quintavalla, A. A New Robust and Efficient Ion?Tagged Proline Catalyst Carrying an Amide Spacer for the Asymmetric Aldol Reaction. Adv. Synth. Catal. 2011, 353, 3234–3240. [Google Scholar] [CrossRef]
- Loh, T.-P.; Feng, L.-C.; Yang, H.-Y.; Yang, J.-Y. l-Proline in an ionic liquid as an efficient and reusable catalyst for direct asymmetric aldol reactions. Tetrahedron Lett. 2002, 43, 8741–8743. [Google Scholar] [CrossRef]
- Kotrusz, P.; Kmentová, I.; Gotov, B.; Toma, Š.; Solčániová, E. Proline-catalysed asymmetric aldol reaction in the room temperature ionic liquid [bmim]PF6. Chem. Commun. 2002, 2510–2511. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Rielaa, S.; Aprile, C.; Meo, P.L.; D’Anna, F.; Noto, R. Supported Ionic Liquids. New Recyclable Materials for theL-Proline-Catalyzed Aldol Reaction. Adv. Synth. Catal. 2006, 348, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Kitazume, T.; Jiang, Z.; Kasai, K.; Mihara, Y.; Suzuki, M. Synthesis of fluorinated materials catalyzed by proline or antibody 38C2 in ionic liquid. J. Fluor. Chem. 2003, 121, 205–212. [Google Scholar] [CrossRef]
- Córdova, A. Direct catalytic asymmetric cross-aldol reactions in ionic liquid media. Tetrahedron Lett. 2004, 45, 3949–3952. [Google Scholar] [CrossRef]
- Miao, W.; Chan, T.-H. Ionic-Liquid-Supported Organocatalyst: Efficient and Recyclable Ionic-Liquid-Anchored Proline for Asymmetric Aldol Reaction. Adv. Synth. Catal. 2006, 348, 1711–1718. [Google Scholar] [CrossRef]
- List, B.; Martínez, A.; Zumbansen, K.; Döhring, A.; Van Gemmeren, M. Improved Conditions for the Proline-Catalyzed Aldol Reaction of Acetone with Aliphatic Aldehydes. Synlett 2014, 25, 932–934. [Google Scholar] [CrossRef]
- Trajković, J.M.; Milanovic, V.D.; Ferjančić, Z.; Saicic, R.N. On the Asymmetric Induction in Proline-Catalyzed Aldol Reactions: Reagent-Controlled Addition Reactions of 2,2-Dimethyl-1,3-dioxane-5-one to Acyclic Chiral α-Branched Aldehydes. Eur. J. Org. Chem. 2017, 6146–6153. [Google Scholar] [CrossRef]
- Rougeot, C.; Situ, H.; Cao, B.H.; Vlachos, V.; Hein, J.E. Automated reaction progress monitoring of heterogeneous reactions: Crystallization-induced stereoselectivity in amine-catalyzed aldol reactions. React. Chem. Eng. 2017, 2, 226–231. [Google Scholar] [CrossRef]
- Brenna, D.; Massolo, E.; Puglisi, A.; Rossi, S.; Celentano, G.; Benaglia, M.; Capriati, V. Towards the development of continuous, organocatalytic, and stereoselective reactions in deep eutectic solvents. Beilstein J. Org. Chem. 2016, 12, 2620–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, B.; Bruckmann, A.; Bolm, C. A Highly Efficient Asymmetric Organocatalytic Aldol Reaction in a Ball Mill. Chem. A Eur. J. 2007, 13, 4710–4722. [Google Scholar] [CrossRef] [PubMed]
- Bruckmann, A.; Krebs, A.; Bolm, C. Organocatalytic reactions: Effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008, 10, 1131. [Google Scholar] [CrossRef]
- Veverková, E.; Modrocká, V.; Sebesta, R. Organocatalyst Efficiency in the α-Aminoxylation and α-Hydrazination of Carbonyl Derivatives in Aqueous Media or in a Ball-Mill. Eur. J. Org. Chem. 2017, 1191–1195. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M.K.; Kumar, M. l-Proline catalysed multicomponent synthesis of 3-amino alkylated indolesvia a Mannich-type reaction under solvent-free conditions. Green Chem. 2012, 14, 290–295. [Google Scholar] [CrossRef]
- Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide?application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy 2004, 7, 42–50. [Google Scholar] [CrossRef]
- Byrne, F.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1034. [Google Scholar] [CrossRef] [Green Version]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854. [Google Scholar] [CrossRef]
- Pihko, P.M.; Laurikainen, K.M.; Usano, A.; Nyberg, A.I.; Kaavi, J.A. Effect of additives on the proline-catalyzed ketone–aldehyde aldol reactions. Tetrahedron 2006, 62, 317–328. [Google Scholar] [CrossRef]
- Kaur, N.; Kishore, D. Synthetic Strategies Applicable in the Synthesis of Privileged Scaffold: 1,4-Benzodiazepine. Synth. Commun. 2014, 44, 1375–1413. [Google Scholar] [CrossRef]
- Ward, D.; Jheengut, V. Proline-catalyzed asymmetric aldol reactions of tetrahydro-4H-thiopyran-4-one with aldehydes. Tetrahedron Lett. 2004, 45, 8347–8350. [Google Scholar] [CrossRef]
- Cai, J.; Wu, Y.-S.; Chen, Y.; Deng, D.-S. Proline-Catalyzed Asymmetric Direct Aldol Reaction Assisted by d-Camphorsulfonic Acid in Aqueous Media. Synlett 2005, 1627–1629. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, Z. (R)- or (S)-Bi-2-naphthol assisted, l-proline catalyzed direct aldol reaction. Tetrahedron Asymmetry 2006, 17, 1671–1677. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, Z. Chiral Diols: A New Class of Additives for Direct Aldol Reaction Catalyzed byl-Proline. J. Org. Chem. 2006, 71, 9510–9512. [Google Scholar] [CrossRef] [PubMed]
- Reis, O.; Eymur, S.; Reis, B.; Demir, A.S. Direct enantioselective aldol reactions catalyzed by a proline–thiourea host–guest complex. Chem. Commun. 2009, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hamdouni, N.; Companyó, X.; Rios, R.; Moyano, A. Substrate-Dependent Nonlinear Effects in Proline-Thiourea-Catalyzed Aldol Reactions: Unraveling the Role of the Thiourea Co-Catalyst. Chem. A Eur. J. 2010, 16, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.S.; Basceken, S. Study of asymmetric aldol and Mannich reactions catalyzed by proline–thiourea host–guest complexes in nonpolar solvents. Tetrahedron Asymmetry 2013, 24, 515–525. [Google Scholar] [CrossRef]
- Martinez-Castaneda, A.; Rodriguez-Solla, H.; Concellón, C.; Del Amo, V. Switching Diastereoselectivity in Proline-Catalyzed Aldol Reactions. J. Org. Chem. 2012, 77, 10375–10381. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Daka, P.; Wang, H. Primary amine-metal Lewis acid bifunctional catalysts: The application to asymmetric direct aldol reactions. Chem. Commun. 2009, 6825. [Google Scholar] [CrossRef] [PubMed]
- Daka, P.; Xu, Z.; Alexa, A.; Wang, H. Primary amine-metal Lewis acid bifunctional catalysts based on a simple bidentate ligand: Direct asymmetric aldol reaction. Chem. Commun. 2011, 47, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, J.; Stodulski, M.; Mlynarski, J. Direct Catalytic Asymmetric Aldol Reactions Assisted by Zinc Complex in the Presence of Water. Adv. Synth. Catal. 2007, 349, 1041–1046. [Google Scholar] [CrossRef]
- Lu, Z.; Mei, H.; Han, J.; Pan, Y. The Mimic of Type II Aldolases Chemistry: Asymmetric Synthesis of β-Hydroxy Ketones by Direct Aldol Reaction. Chem. Boil. Drug Des. 2010, 76, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Kitamura, M.; Yamada, Y.; Aoki, S. Chiral Catalysts Dually Functionalized with Amino Acid and Zn2+Complex Components for Enantioselective Direct Aldol Reactions Inspired by Natural Aldolases: Design, Synthesis, Complexation Properties, Catalytic Activities, and Mechanistic Study. Chem. A Eur. J. 2009, 15, 10570–10584. [Google Scholar] [CrossRef]
- Kofoed, J.; Darbre, T.; Reymond, J. Dual mechanism of zinc-proline catalyzed aldol reactions in water. Chem. Commun. 2006, 1482. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, J.; Machuqueiro, M.; Reymond, J.; Darbre, T. Zinc–proline catalyzed pathway for the formation of sugars. Chem. Commun. 2004, 1540. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, R.; Kofoed, J.; Machuqueiro, M.; Darbre, T. A Selective Direct Aldol Reaction in Aqueous Media Catalyzed byZinc-Proline. Eur. J. Org. Chem. 2005, 5268–5276. [Google Scholar] [CrossRef]
- Kofoed, J.; Reymond, J.; Darbre, T. Prebiotic carbohydrate synthesis: Zinc–proline catalyzes direct aqueous aldol reactions of α-hydroxy aldehydes and ketones. Org. Biomol. Chem. 2005, 3, 1850. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, K.; Sakamoto, S.; Kudo, K. Direct asymmetric aldol reaction in aqueous media using polymer-supported peptide. Tetrahedron Lett. 2005, 46, 8185–8187. [Google Scholar] [CrossRef]
- Penhoat, M.; Barbry, D.; Rolando, C. Direct asymmetric aldol reaction co-catalyzed by l-proline and group 12 elements Lewis acids in the presence of water. Tetrahedron Lett. 2011, 52, 159–162. [Google Scholar] [CrossRef]
- Karmakar, A.; Maji, T.; Wittmann, S.; Reiser, O. L-Proline/CoCl2-Catalyzed Highly Diastereo- and Enantioselective Direct Aldol Reactions. Chem. A Eur. J. 2011, 17, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2019, 27, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total. Environ. 2020, 709, 135895. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Lindström, U.M. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef] [PubMed]
- Sinou, D. Asymmetric Organometallic-Catalyzed Reactions in Aqueous Media. Adv. Synth. Catal. 2002, 344, 221–237. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process. Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Clegg, W.; Harrington, R.W.; North, M.; Pizzato, F.; Villuendas, P. Cyclic carbonates as sustainable solvents for proline-catalysed aldol reactions. Tetrahedron Asymmetry 2010, 21, 1262–1271. [Google Scholar] [CrossRef]
- Tan, R.; Li, C.; Luo, J.; Kong, Y.; Zheng, W.; Yin, D. An effective heterogeneous l-proline catalyst for the direct asymmetric aldol reaction using graphene oxide as support. J. Catal. 2013, 298, 138–147. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, X.; Wang, X.; Xiao, S.; Wang, Y. An Efficient Ionic Liquid Additive for Proline-catalyzed Direct Asymmetric Aldol Reactions between Cyclic Ketones and Aromatic Aldehydes. Chem. Lett. 2009, 38, 576–577. [Google Scholar] [CrossRef]
- Rodriguez, B.; Rantanen, T.; Bolm, C. Solvent-Free Asymmetric Organocatalysis in a Ball Mill. Angew. Chem. Int. Ed. 2006, 45, 6924–6926. [Google Scholar] [CrossRef] [PubMed]
- Obregon, A.; Milán, M.; Juaristi, E. Improving the Catalytic Performance of (S)-Proline as Organocatalyst in Asymmetric Aldol Reactions in the Presence of Solvate Ionic Liquids: Involvement of a Supramolecular Aggregate. Org. Lett. 2017, 19, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castañeda, A.; Poladura, B.; Rodriguez-Solla, H.; Concellón, C.; Del Amo, V. Direct Aldol Reactions Catalyzed by a Heterogeneous Guanidinium Salt/Proline System under Solvent-Free Conditions‡. Org. Lett. 2011, 13, 3032–3035. [Google Scholar] [CrossRef] [PubMed]
- Szőllősi, G.; Fekete, M.; Gurka, A.A.; Bartók, M. Reversal of Enantioselectivity in Aldol Reaction: New Data on Proline/γ-Alumina Organic–Inorganic Hybrid Catalysts. Catal. Lett. 2013, 144, 478–486. [Google Scholar] [CrossRef]
- North, M.; Villuendas, P. A Chiral Solvent Effect in Asymmetric Organocatalysis. Org. Lett. 2010, 12, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Aratake, S.; Itoh, T.; Okano, T.; Sumiya, T.; Shoji, M. Dry and wet prolines for asymmetric organic solvent-free aldehyde–aldehyde and aldehyde–ketone aldol reactions. Chem. Commun. 2007, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Wu, Y.; Zhao, X.; Wang, J.; Zhang, L.; Cui, Y. Polymerization of l-proline functionalized styrene and its catalytic performance as a supported organocatalyst for direct enantioselective aldol reaction. Tetrahedron Asymmetry 2016, 27, 740–746. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Wang, Z.; Yan, J. Merrifield Resin Supported Ionic Liquids/l-Proline as Efficient and Recyclable Catalyst Systems for Asymmetric Aldol Reaction. Synthesis 2009, 3744–3750. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Wang, X.; Zhang, F.; Zhong, X.; Dong, Z.; Ma, J. Core–shell silica magnetic microspheres supported proline as a recyclable organocatalyst for the asymmetric aldol reaction. J. Mol. Catal. A Chem. 2012, 363, 404–410. [Google Scholar] [CrossRef]
- Yacob, Z.; Nan, A.; Liebscher, J. Proline-Functionalized Magnetic Core-Shell Nanoparticles as Efficient and Recyclable Organocatalysts for Aldol Reactions. Adv. Synth. Catal. 2012, 354, 3259–3264. [Google Scholar] [CrossRef]
- Liebscher, J.; Shah, J.; Khan, S.; Blumenthal, H. 1,2,3-Triazolium-Tagged Prolines and Their Application in Asymmetric Aldol and Michael Reactions. Synthesis 2009, 3975–3982. [Google Scholar] [CrossRef]
- Kucherenko, A.S.; Struchkova, M.I.; Zlotin, S.G. The (S)-Proline/Polyelectrolyte System: An Efficient, Heterogeneous, Reusable Catalyst for Direct Asymmetric Aldol Reactions. Eur. J. Org. Chem. 2006, 2000–2004. [Google Scholar] [CrossRef]
- Ibrahem, I.; Zou, W.; Xu, Y.; Córdova, A. Amino Acid-Catalyzed Asymmetric Carbohydrate Formation: Organocatalytic One-StepDe Novo Synthesis of Keto and Amino Sugars. Adv. Synth. Catal. 2006, 348, 211–222. [Google Scholar] [CrossRef]
- Suri, J.T.; Mitsumori, S.; Albertshofer, K.; Tanaka, F.; Barbas, C.F. Dihydroxyacetone Variants in the Organocatalytic Construction of Carbohydrates: Mimicking Tagatose and Fuculose Aldolases. J. Org. Chem. 2006, 71, 3822–3828. [Google Scholar] [CrossRef] [PubMed]
- Grondal, C.; Enders, D. Direct asymmetric organocatalytic de novo synthesis of carbohydrates. Tetrahedron 2006, 62, 329–337. [Google Scholar] [CrossRef]
- Majewski, M.; Niewczas, I.; Palyam, N. Acids as Proline Co-catalysts in the Aldol Reaction of 1,3-Dioxan-5-ones. Synlett 2006, 2387–2390. [Google Scholar] [CrossRef]
- Ibrahem, I.; Córdova, A. Amino acid catalyzed direct enantioselective formation of carbohydrates: One-step de novo synthesis of ketoses. Tetrahedron Lett. 2005, 46, 3363–3367. [Google Scholar] [CrossRef]
- Gong, Z.; Wei, C.; Shi, Y.; Zheng, Q.; Song, Z.; Liu, Z. Novel chiral bifunctional l-thiazoline-amide derivatives: Design and application in the direct enantioselective aldol reactions. Tetrahedron 2014, 70, 1827–1835. [Google Scholar] [CrossRef]
- Miura, T.; Kasuga, H.; Imai, K.; Ina, M.; Tada, N.; Imai, N.; Itoh, A. Highly efficient asymmetric aldol reaction in brine using a fluorous sulfonamide organocatalyst. Org. Biomol. Chem. 2012, 10, 2209. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Y.; Gai, X.; Zeng, X. Highly Enantio- and Diastereoselective l-Proline Derived Acetylglucose Amide Catalyzed Aldol Reaction of Ketones to Aldehydes under Solvent-Free Conditions. Synlett 2015, 26, 2858–2862. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.; Gleave, D.M.; Nowak, P. 1,3-Dioxan-5-ones: Synthesis, deprotonation, and reactions of their lithium enolates. Can. J. Chem. 1995, 73, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Ying, A.; Liu, S.; Li, Z.; Chen, G.; Yang, J.; Yan, H.; Xu, S. Magnetic Nanoparticles-Supported Chiral Catalyst with an Imidazolium Ionic Moiety: An Efficient and Recyclable Catalyst for Asymmetric Michael and Aldol Reactions. Adv. Synth. Catal. 2016, 358, 2116–2125. [Google Scholar] [CrossRef]
- Sai, M.; Yamamoto, H. Chiral Brønsted Acid as a True Catalyst: Asymmetric Mukaiyama Aldol and Hosomi–Sakurai Allylation Reactions. J. Am. Chem. Soc. 2015, 137, 7091–7094. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Marcus, R.A. On the Theory of Organic Catalysis “on Water”. J. Am. Chem. Soc. 2007, 129, 5492–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, A.; Boto, R.A.; Dingwall, P.; Contreras-Garcia, J.; Harvey, M.J.; Mason, N.; Rzepa, H. The Houk–List transition states for organocatalytic mechanisms revisited. Chem. Sci. 2014, 5, 2057–2071. [Google Scholar] [CrossRef] [Green Version]
- Patil, M.P.; Sunoj, R.B. Insights on Co-Catalyst-Promoted Enamine Formation between Dimethylamine and Propanal through Ab Initio and Density Functional Theory Study. J. Org. Chem. 2007, 72, 8202–8215. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Seguin, T.J.; Guan, Y.; Doney, A. Noncovalent Interactions in Organocatalysis and the Prospect of Computational Catalyst Design. Acc. Chem. Res. 2016, 49, 1061–1069. [Google Scholar] [CrossRef] [PubMed]


| R (2) | Solvent | t [h] | 3 Conversion [%] 2 | ee [%] 3 | anti/syn2 |
|---|---|---|---|---|---|
| 4-NO2Ph (2a) | MeOH/H2O | 19 | >99 | 98 | 93:7 |
| H2O | 19 | 25 | 99 | 95:5 | |
| MeOH | 19 | >99 | 76 | 59:41 | |
| 4-CNPh (2b) | MeOH/H2O | 19 | 97 | 98 | 95:5 |
| H2O | 19 | 80 | 99 | 95:5 | |
| MeOH | 19 | >99 | 87 | 82:18 | |
| 4-ClPh (2c) | MeOH/H2O | 19 | 43 | 99 | 97:3 |
| H2O | 19 | 5 | >99 | nd | |
| MeOH | 19 | 64 | 98 | 85:15 | |
| Ph (2d) 4 | MeOH/H2O | 30 | 58 | 97 | 88:12 |
| H2O | 30 | 20 | >99 | >99:1 | |
| MeOH | 30 | 64 | 83 | 72:28 |
| R (2) | t [h] | 3 Conversion [%] 2 | ee [%] 3 | anti/syn2 |
|---|---|---|---|---|
| C6F5 (2e) | 19 | >99 | 97 | >99:1 |
| 2-NO2Ph (2f) | 19 | 93 | 95 | 95:5 |
| 4-BrPh (2g) | 19 | 41 | 99 | 98:2 |
| 2-naphthyl (2h) | 24 | 37 | 93 | 91:9 |
| 4-MeOPh (2i) 4 | 70 | 18 | 90 | 86:14 |
| R (2) | MeOH/H2O [μL/μL] | t [h] | 3 Conversion [%] 2 | ee [%] 3 | anti/syn2 |
|---|---|---|---|---|---|
| 4-NO2Ph (2a) | 20/10 | 4 | 3aa, 47 | 97 | 94:6 |
| 40/10 | 4 | 3aa, 82 | 98 | 92:8 | |
| 20 + 20 4/10 | 4 | 3aa, 84 | 97 | 94:6 | |
| 4-CNPh (2b) | 20/10 | 4 | 3ab, 65 | 97 | 95:5 |
| 40/10 | 4 | 3ab, 77 | 95 | 94:6 | |
| 4-ClPh (2c) | 20/10 | 19 | 3ac, 43 | 99 | 97:3 |
| 40/10 | 19 | 3ac, 64 | 98 | 95:5 | |
| Ph (2d) | 20/10 5 | 30 | 3ad, 58 | 97 | 88:12 |
| 40/10 | 30 | 3ad, 75 | 96 | 90:10 | |
| C6F5 (2e) | 20/10 | 4 | 3ae, 63 | 97 | >99:1 |
| 40/10 | 4 | 3ae, 67 | 96 | >99:1 | |
| 2-NO2Ph (2f) | 20/10 | 4 | 3af, 34 | 97 | 98:2 |
| 40/10 | 4 | 3af, 59 | 97 | 97:3 | |
| 4-BrPh (2g) | 20/10 | 19 | 3ag, 41 | 99 | 98:2 |
| 40/10 | 19 | 3ag, 88 | 96 | 94:6 | |
| 2-naphthyl (2h) | 20/10 | 24 | 3ah, 37 | 93 | 91:9 |
| 40/10 | 24 | 3ah, 72 | 93 | 92:8 | |
| 4-MeOPh (2i) 5 | 20/10 | 68 | 3ai, 18 | 90 | 86:14 |
| 40/10 | 68 | 3ai, 43 | 89 | 80:20 | |
| i-Pr (2j) 5 | 40/10 | 72 | 3aj, 63 | >99 | >99:1 |
| 4-CF3Ph (2k) | 40/10 | 4 | 3ak, 78 | 97 | 97:3 |
| 2-thiophenyl (2l) 5 | 40/10 | 48 | 3al, 65 | 88 | 84:16 |
| 4-CH3Ph (2m) | 40/10 | 40 | 3am, 64 | 94 | 87:13 |
| R (2) | 1a [eq.] | t [h] | 3 Conversion [%] 2 | ee [%] 3 | anti/syn2 |
|---|---|---|---|---|---|
| 4-NO2Ph (2a) | 5 | 4 | 82 | 98 | 92:8 |
| 5 | 4 | 84 4 | 97 | 94:6 | |
| 2 | 19 | 99 | 95 | 92:8 | |
| 1.05 | 19 | 92 | 97 | 90:10 | |
| 4-CNPh (2b) | 5 | 4 | 77 | 95 | 94:6 |
| 2 | 24 | 98 | 91 | 90:10 | |
| C6F5 (2e) | 5 | 4 | 67 | 97 | >99:1 |
| 2 | 24 | >99 | 92 | >99:1 | |
| 2-NO2Ph (2f) | 5 | 4 | 59 | 97 | 97:3 |
| 2 | 24 | 92 | 93 | 94:6 | |
| 4-BrPh (2g) | 5 | 19 | 88 | 96 | 94:6 |
| 2 | 24 | 97 | 91 | 90:10 | |
| 4-CF3Ph (2k) | 5 | 4 | 78 | 97 | 97:3 |
| 3 | 20 | 96 | 96 | 95:5 | |
| 2 | 24 | 93 | 94 | 93:7 |
| (1) | R (2) | t [h] | 3, Conversion [%] 2 | ee [%] 3 | anti/syn2 |
|---|---|---|---|---|---|
 (1b) | 4-NO2Ph (2a) | 4 | 3ba, >99 | 94 | 61:39 |
| 4-NO2Ph (2a) | 19 4 | 3ba, 91 | 94 | 78:22 | |
| 4-BrPh (2g) | 19 | 3bg, 64 | 93 | 75:25 | |
| Ph (2d) | 30 | 3bd, 70 | 93 | 73:27 | |
 (1c) | 4-BrPh (2g) | 24 | 3cg, 90 | 86 | 78:22 |
| Ph (2d) | 48 | 3cd, 82 | 86 | 77:23 |
| Aldehyde Addition Rate | t [h] | Conversion [%] 2 | anti/syn2 | ee [%] 3 |
|---|---|---|---|---|
| 45 min | 23 | 72 | 86:14 | 96 |
| 28 | 78 | 87:13 | - | |
| 47 | 83 | 85:15 | 94 | |
| 6 h | 23 | 71 | 87:13 | - |
| 28 | 80 | 85:15 | - | |
| 47 | 85 | 84:16 | - |
| R (2) | t [h] | Conversion [%] 2 | anti/syn2 |
|---|---|---|---|
| Ph (2d) | 24 | 75 | 86:14 |
| 46 | 81 | 84:16 | |
| 71 | 85 | 79:21 | |
| 94 | 85 | 71:29 | |
| 4-CH3Ph (2m) | 23 | 53 | 90:10 |
| 45 | 65 | 87:13 | |
| 71 | 74 | 84:16 | |
| 138 | 75 | 79:21 | |
| 4-MeOPh (2i) 3 | 68 | 43 | 80:20 |
| 115 | 49 | 72:28 | |
| 164 | 52 | 66:34 |
| Method | Conditions | Final Volume (mL) | Crude Analysis 2 |
|---|---|---|---|
| A | Reaction mixture = 18 mL (22.8 mmol) filtered on a silica-pad, mobile phase = EtOAc | 242 | Conv. = 87% dr = 84:16 |
| B | Reaction mixture = 18 mL (22.8 mmol) diluted with EtOAc, quenched with aqueous NH4Cl, extracted with EtOAc, dried with Na2SO4 (washed with EtOAc) | 90 | Conv. = 86% dr = 83:17 |
| C | Reaction mixture = 10 mL (12.6 mmol) diluted with EtOAc and placed at −15 °C for 36 h. 1° vacuum filtration. At −15 °C for 36 h. 2° vacuum filtration. Solution dried with Na2SO4 (washed with EtOAc) | 46 | Conv. = 89% dr = 84:16 Proline recovery: 113.3 mg (78%) |
| D | Reaction mixture = 10 mL (12.6 mmol) diluted with Et2O and placed at −15 °C for 36 h. 1° vacuum filtration. At −15 °C for 36 h. 2° vacuum filtration. Solution dried with Na2SO4 (washed with Et2O) | 52 | Conv. = 90% dr = 85:15 Proline recovery: 124.8 mg (86%) |
| E | Reaction mixture = 10 mL (12.6 mmol) diluted with DCM and placed at −15 °C for 36 h. Two liquid phases obtained, dried with Na2SO4 (washed with DCM) | 36 | Conv. = 88% dr = 85:15 |
| F | Reaction mixture = 10 mL (12.6 mmol) diluted with n-hexane and placed at −15 °C for 36 h. Two liquid phases obtained, dried with Na2SO4 (washed with n-hexane) | 43 | Conv. = 89% dr = 86:14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emma, M.G.; Tamburrini, A.; Martinelli, A.; Lombardo, M.; Quintavalla, A.; Trombini, C. A Simple and Efficient Protocol for Proline-Catalysed Asymmetric Aldol Reaction. Catalysts 2020, 10, 649. https://doi.org/10.3390/catal10060649
Emma MG, Tamburrini A, Martinelli A, Lombardo M, Quintavalla A, Trombini C. A Simple and Efficient Protocol for Proline-Catalysed Asymmetric Aldol Reaction. Catalysts. 2020; 10(6):649. https://doi.org/10.3390/catal10060649
Chicago/Turabian StyleEmma, Marco Giuseppe, Alice Tamburrini, Ada Martinelli, Marco Lombardo, Arianna Quintavalla, and Claudio Trombini. 2020. "A Simple and Efficient Protocol for Proline-Catalysed Asymmetric Aldol Reaction" Catalysts 10, no. 6: 649. https://doi.org/10.3390/catal10060649