Effects of Cardiotoxins from Naja oxiana Cobra Venom on Rat Heart Muscle and Aorta: A Comparative Study of Toxin-Induced Contraction Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
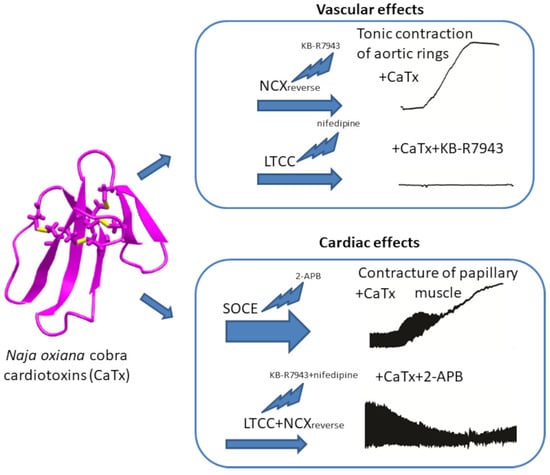
2.1. Comparison of Cardiotoxins Effects on the Rat Papillary Muscle and Aorta
2.2. Comparison of Cardiotoxins Time-Dependent Effects on Contractility and Diastolic Tension in Rat Papillary Muscle
2.3. Effects of Cardiotoxins on Force-Frequency Relationship in Papillary Muscle
2.4. Molecular Mechanisms of the Papillary Muscle Contractile Response Induced by Cardiotoxins
2.5. Molecular Mechanisms of the Aorta Rings Contractile Response Induced by Cardiotoxins
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Animal Handling
4.3. Contractility of Papillary Muscles
4.4. Contractility of Aortic Rings
4.5. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaisakul, J.; Isbister, G.K.; Konstantakopoulos, N.; Tare, M.; Parkington, H.C.; Hodgson, W.C. In vivo and in vitro cardiovascular effects of Papuan taipan (Oxyuranus scutellatus) venom: Exploring ”sudden collapse”. Toxicol. Lett. 2012, 213, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Vuong, N.T.; Jackson, T.N.W.; Wright, C.E. Role of Phospholipases A2 in Vascular Relaxation and Sympatholytic Effects of Five Australian Brown Snake, Pseudonaja spp., Venoms in Rat Isolated Tissues. Front. Pharmacol. 2021, 12, 754304. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Koh, C.Y. Snake venom three-finger toxins and their potential in drug development targeting cardiovascular diseases. Biochem. Pharmacol. 2020, 181, 114105. [Google Scholar] [CrossRef] [PubMed]
- Averin, A.S.; Utkin, Y.N. Cardiovascular Effects of Snake Toxins: Cardiotoxicity and Cardioprotection. Acta Nat. 2021, 13, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.K.; Jayaraman, G.; Lee, C.S.; Arunkumar, A.I.; Sivaraman, T.; Samuel, D.; Yu, C. Snake venom cardiotoxins-structure, dynamics, function and folding. J. Biomol. Struct. Dyn. 1997, 15, 431–463. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.C.; Chiu, T.H.; Chiu, P.J.; Tseng, T.C.; Lee, S.Y. Pharmacological properties of cardiotoxin isolated from Formosan cobra venom. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968, 259, 360–374. [Google Scholar] [CrossRef]
- Sun, J.J.; Walker, M.J. Actions of cardiotoxins from the southern Chinese cobra (Naja naja atra) on rat cardiac tissue. Toxicon 1986, 24, 233–245. [Google Scholar] [CrossRef]
- Wang, H.X.; Lau, S.Y.; Huang, S.J.; Kwan, C.Y.; Wong, T.M. Cobra venom cardiotoxin induces perturbations of cytosolic calcium homeostasis and hypercontracture in adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 1997, 29, 2759–2770. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Shrivastava, I.H.; Israilov, F.S.; Kim, A.A.; Rylova, K.A.; Zhang, B.; Dagda, R.K. Naja naja oxiana Cobra Venom Cytotoxins CTI and CTII Disrupt Mitochondrial Membrane Integrity: Implications for Basic Three-Fingered Cytotoxins. PLoS ONE 2015, 10, e0129248. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Shrivastava, I.H.; Hanlon, P.; Dagda, R.K.; Gasanoff, E.S. Molecular Mechanism by which Cobra Venom Cardiotoxins Interact with the Outer Mitochondrial Membrane. Toxins 2020, 12, 425. [Google Scholar] [CrossRef]
- Dubovskii, P.V.; Lesovoy, D.M.; Dubinnyi, M.A.; Konshina, A.G.; Utkin, Y.N.; Efremov, R.G.; Arseniev, A.S. Interaction of three-finger toxins with phospholipid membranes: Comparison of S- and P-type cytotoxins. Biochem. J. 2005, 387, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Monette, R.; Lee, S.C.; Morley, P.; Wu, W.G. Cobra cardiotoxin-induced cell death in fetal rat cardiomyocytes and cortical neurons: Different pathway but similar cell surface target. Toxicon 2005, 46, 430–440. [Google Scholar] [CrossRef]
- Grishin, E.V.; Sukhikh, A.P.; Adamovich, T.B.; Ovchinnikov, Y.A. Isolation, properties, and amino acid sequence of two cytotoxins from the venom of the Central Asian cobra Naja naja oxiana. Bioorg. Khim. 1976, 2, 1018–1034. [Google Scholar]
- Cher, C.D.N.; Armugam, A.; Zhu, Y.Z.; Jeyaseelan, K. Molecular basis of cardiotoxicity upon cobra envenomation. Cell. Mol. Life Sci. 2005, 62, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Kornisiuk, E.; Bradley, K.N.; Cerveñansky, C.; Durán, R.; Adrover, M.; Sánchez, G.; Jerusalinsky, D. Effects of muscarinic toxins MT1 and MT2 from green mamba on different muscarinic cholinoceptors. Neurochem. Res. 2002, 27, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, N.; Pung, Y.F.; Zhu, Y.Z.; Wong, P.T.H.; Kumar, P.P.; Kini, R.M. Beta-cardiotoxin: A new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity. FASEB J. 2007, 21, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Rouget, C.; Quinton, L.; Maïga, A.; Gales, C.; Masuyer, G.; Malosse, C.; Chamot-Rooke, J.; Thai, R.; Mourier, G.; de Pauw, E.; et al. Identification of a novel snake peptide toxin displaying high affinity and antagonist behaviour for the α2-adrenoceptors. Br. J. Pharmacol. 2010, 161, 1361–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averin, A.S.; Astashev, M.E.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N. Cardiotoxins from Cobra Naja oxiana Change the Force of Contraction and the Character of Rhythmoinotropic Phenomena in the Rat Myocardium. Dokl. Biochem. Biophys. 2019, 487, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Wu, C.K.; Sun, J.J. Positive inotropic and toxic action of direct lytic factor on isolated working guinea pig hearts. Zhongguo Yao Li Xue Bao 1991, 12, 125–131. [Google Scholar] [PubMed]
- Loots, J.M.; Meij, H.S.; Meyer, B.J. Effects of Naja nivea venom on nerve, cardiac and skeletal muscle activity of the frog. Br. J. Pharmacol. 1973, 47, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, K.H.; Kwan, C.Y.; Huang, S.J.; Bourreau, J.P. Dual effect of cobra cardiotoxin on vascular smooth muscle and endothelium. Zhongguo Yao Li Xue Bao 1998, 19, 197–202. [Google Scholar] [PubMed]
- Kwan, C.Y.; Kwan, T.K.; Huang, S.J. Effect of calcium on the vascular contraction induced by cobra venom cardiotoxin. Clin. Exp. Pharmacol. Physiol. 2002, 29, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Lin-Shiau, S.Y.; Huang, H.C.; Lee, C.Y. A comparison of the actions of cobra cardiotoxin and scorpion toxin II on the guinea-pig taenia coli. Toxicon 1986, 24, 131–139. [Google Scholar] [CrossRef]
- Harvey, A.L.; Marshall, R.J.; Karlsson, E. Effects of purified cardiotoxins from the Thailand cobra (Naja naja siamensis) on isolated skeletal and cardiac muscle preparations. Toxicon 1982, 20, 379–396. [Google Scholar] [CrossRef]
- Chen, K.M.; Guan, Y.Y.; Sun, J.J. Effects of direct lytic factors from southern Chinese cobra venom on Ca2+ movement in rabbit aorta strip. Zhongguo Yao Li Xue Bao 1993, 14, 500–504. [Google Scholar] [PubMed]
- Huang, S.J.; Kwan, C.Y. Inhibition by multivalent cations of contraction induced by Chinese cobra venom cardiotoxin in guinea pig papillary muscle. Life Sci. 1996, 59, PL55–PL60. [Google Scholar] [CrossRef]
- Chong, H.P.; Tan, K.Y.; Tan, C.H. Cytotoxicity of Snake Venoms and Cytotoxins From Two Southeast Asian Cobras (Naja sumatrana, Naja kaouthia): Exploration of Anticancer Potential, Selectivity, and Cell Death Mechanism. Front. Mol. Biosci. 2020, 7, 583587. [Google Scholar] [CrossRef]
- Huang, J.L.; Trumble, W.R. Cardiotoxin from cobra venom affects the Ca-Mg-ATPase of cardiac sarcolemmal membrane vesicles. Toxicon 1991, 29, 31–41. [Google Scholar] [CrossRef]
- Nayler, W. The effect of a cardiotoxic component of the venom of the Indian cobra (Naja nigricollis) on the subcellular structure and function of heart muscle. J. Mol. Cell. Cardiol. 1976, 8, 341–356. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Kalinin, A.L.; Selistre-de-Araujo, H.S.; Vasconcelos, E.S.; Rantin, F.T. Alternagin-C (ALT-C), a disintegrin-like protein from Rhinocerophis alternatus snake venom promotes positive inotropism and chronotropism in fish heart. Toxicon 2016, 110, 1–11. [Google Scholar] [CrossRef]
- Endoh, M. Force-frequency relationship in intact mammalian ventricular myocardium: Physiological and pathophysiological relevance. Eur. J. Pharmacol. 2004, 500, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Stuyvers, B.D.; McCulloch, A.D.; Guo, J.; Duff, H.J.; ter Keurs, H.E.D.J. Effect of stimulation rate, sarcomere length and Ca(2+) on force generation by mouse cardiac muscle. J. Physiol. 2002, 544, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000, 87, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.E.; Tripolitis, L.; Beech, J. Species difference in modulation of calcium release by Naja naja kaouthia snake venom cardiotoxin in terminal cisternae from human and equine skeletal muscle. Toxicon 1993, 31, 43–51. [Google Scholar] [CrossRef]
- Tzeng, W.F.; Chen, Y.H. Suppression of snake-venom cardiotoxin-induced cardiomyocyte degeneration by blockage of Ca2+ influx or inhibition of non-lysosomal proteinases. Biochem. J. 1988, 256, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Debnath, A.; Saha, A.; Gomes, A.; Biswas, S.; Chakrabarti, P.; Giri, B.; Biswas, A.K.; Gupta, S.D.; Gomes, A. A lethal cardiotoxic-cytotoxic protein from the Indian monocellate cobra (Naja kaouthia) venom. Toxicon 2010, 56, 569–579. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [Green Version]
- Avila-Medina, J.; Mayoral-Gonzalez, I.; Dominguez-Rodriguez, A.; Gallardo-Castillo, I.; Ribas, J.; Ordoñez, A.; Rosado, J.A.; Smani, T. The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells. Front. Physiol. 2018, 9, 257. [Google Scholar] [CrossRef]
- Ishida, H.; Saito, S.-Y.; Hishinuma, E.; Ishikawa, T. Differential Contribution of Nerve-Derived Noradrenaline to High K+-Induced Contraction Depending on Type of Artery. Biol. Pharm. Bull. 2017, 40, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Dubovskii, P.V.; Lesovoy, D.M.; Dubinnyi, M.A.; Utkin, Y.N.; Arseniev, A.S. Interaction of the P-type cardiotoxin with phospholipid membranes. Eur. J. Biochem. 2003, 270, 2038–2046. [Google Scholar] [CrossRef] [Green Version]
- Dubovskii, P.V.; Dubinnyi, M.A.; Volynsky, P.E.; Pustovalova, Y.E.; Konshina, A.G.; Utkin, Y.N.; Arseniev, A.S.; Efremov, R.G. Impact of membrane partitioning on the spatial structure of an S-type cobra cytotoxin. J. Biomol. Struct. Dyn. 2018, 36, 3463–3478. [Google Scholar] [CrossRef] [PubMed]
- Nakipova, O.V.; Averin, A.S.; Evdokimovskii, E.V.; Pimenov, O.Y.; Kosarski, L.; Ignat’ev, D.; Anufriev, A.; Kokoz, Y.M.; Reyes, S.; Terzic, A.; et al. Store-operated Ca2+ entry supports contractile function in hearts of hibernators. PLoS ONE 2017, 12, e0177469. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averin, A.S.; Nenov, M.N.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Effects of Cardiotoxins from Naja oxiana Cobra Venom on Rat Heart Muscle and Aorta: A Comparative Study of Toxin-Induced Contraction Mechanisms. Toxins 2022, 14, 88. https://doi.org/10.3390/toxins14020088
Averin AS, Nenov MN, Starkov VG, Tsetlin VI, Utkin YN. Effects of Cardiotoxins from Naja oxiana Cobra Venom on Rat Heart Muscle and Aorta: A Comparative Study of Toxin-Induced Contraction Mechanisms. Toxins. 2022; 14(2):88. https://doi.org/10.3390/toxins14020088
Chicago/Turabian StyleAverin, Alexey S., Miroslav N. Nenov, Vladislav G. Starkov, Victor I. Tsetlin, and Yuri N. Utkin. 2022. "Effects of Cardiotoxins from Naja oxiana Cobra Venom on Rat Heart Muscle and Aorta: A Comparative Study of Toxin-Induced Contraction Mechanisms" Toxins 14, no. 2: 88. https://doi.org/10.3390/toxins14020088