Toxic Effects Produced by Anatoxin-a under Laboratory Conditions: A Review
Abstract
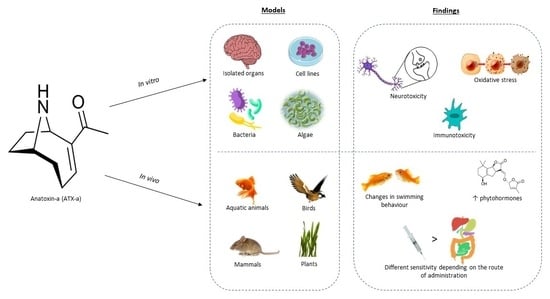
:1. Introduction
2. In Vitro Toxicity Studies
3. In Vivo Experimental Studies
| Experimental Model | Experimental Conditions | Assays Performed | Main Results | References |
|---|---|---|---|---|
| Aquatic Animals | ||||
| Goldfish (and other species, see birds and mammmals) | Oral or i.p. doses of Anabaena flos-aquae NRC-44-1 or immersion in an aqueous medium containing 6 µg/mL toxin extract for 8 h | Clinical observations | Death was produced by respiratory arrest after 12–14 min when administration was orally or i.p. No adverse effects were observed when fish were placed in an aqueous medium containing the toxin. | [69] |
| Goldfish (and other species, see birds and mammmals) | I.p. injection or oral doses of Anabaena flos-aquae NRC-44-1 containing ATX-a | Determination of LD90 | When administration was oral, goldfish were the most sensitive species to ATX-a (LD90 = 120 mg/kg). The i.p. LD90 was half that of the oral dose (LD90 = 60 mg/kg). | [68] |
| Brine shrimp (Artemia salina) | 25 or 50 µg/mL of pure ATX-a, 20 µg ATX-a per mg of nontoxic Anabaena or Anabaena strains containing ATX-a | Toxicity determination by Artemia salina biotest | Concentration up to 50 µg/mL of pure ATX-a were not toxic to Artemia larvae, although when ATX-a was mixed with nontoxic Anabaena, an increase in the death percentage of the larvae was observed. This result may indicate that ATX-a was not the responsible compound of that toxicity. Abnormal movements were observed with Anabaena strains containing ATX-a. | [70] |
| Brine shrimp (Artemia salina) | 0–100 mg/L Anabaena strains containing ATX-a or cyanobacterial bloom | Toxicity determination by Artemia salina biotest | ATX-a only produced abnormal swimming in the A. salina bioassay, whereas Anabaena strains containing ATX-a caused mortality (LC50 = 2–14 mg/L). | [71] |
| Zebrafish embryos | Concentrations of 40, 200 or 400 µg/L ATX-a and exposure to crude extracts of cyanobacteria | Heart rate measurement and malformation observation | The highest concentration produced temporary alterations in heart rates. No chronic effects were observed. No effects were observed with the crude extract in which ATX-a was detected. | [72] |
| Embryos of toads (Bufo arenarum) | Amphibian stage 18 embryos were exposed to 0.03, 0.3, 3.0 or 30 mg/L ATX-a for 10 days and stage 25 embryos were exposed to 30 mg/L ATX-a for 10 days. | Embryo-larval toxicity test (AMPHITOX) | Toad embryos shown a concentration-dependent transient narcosis, oedema and loss of equilibrium as adverse effects, and a mortality of 100% at the highest concentration in both groups 6–13 days post-exposure. | [73] |
| Cyprinus carpio | 105 cel/mL or 107 cel/mL of Anabaena containing ATX-a for 4 days | Study of behavioral and bioaccumulation of toxin by HPLC | Treated carps showed behavior alterations. The highest cyanobacteria concentration caused the death of all fish, whereas with the small one, no deaths were observed. The highest level of toxin detected in the whole fish was 0.768 µg/g of carp weight. | [74] |
| Fertilized eggs from Cyprinus carpio | Fertilized eggs were incubated over 4 days with cyanobacterial cell extract of Anabaena sp. (6.6 × 105–8.3 × 104 cell/L that correspond to 83.3–666 µg/L ATX-a) or pure ATX-a (80–640 µg/L) | Registration of mortality analysis of hatching rate and skeletal malformations at 4, 9 and 24 h, and every 24 h for 8 days after the first exposure | Pure toxin only produced a decrease in larval length at the highest concentration. However, concentration-dependent adverse effects were observed with the cyanobacterial extract, producing 100% mortality at the highest concentration. | [75] |
| Common carp | 25 µg/L of ATX-a for 5 days by inmersion | Cytotoxicity by bioluminescent assay and proliferation by DNA fragmentation | Decreased ATP levels were not observed. A reduction in GSH levels and proliferation of T and B lymphocytes in pronephros and blood was produced. | [56] |
| Rainbow trout (Oncorhynchus mykiss) | Range-finding bioassay: Single dose of 0.005–5 µg/g ATX-a by i.p. injection Main test: 0.08–0.31 ATX-a by i.p. injection | Determination of LD50 Measurement of enzymatic biomarkers in muscle or liver | Survival after exposure to the lowest doses of the toxin. Death at 30 and 17 min after treatment with 0.5 and 5 µg/g, respectively. The LD50 determined was 0.36 µg/g. An increase in AChE and LDH activities in muscle and GST and EROD activities in liver were observed. The rise of these activities in the liver indicated the involvement of phase I and II biotransformation in ATX-a detoxification. | [30] |
| Zebrafish | Dose of 0.8 µg/g b.w. (±)ATX-a by i.p. injection | Study of behavior and comparison of proteome in brain and muscle between gender by 2DE analysis and mass spectrometry | Fish showed behavior alterations. Males showed more increase in the abundance of proteins than females. Also, differences in protein expression were observed between gender. Proteins that were altered play functions in stress response, detoxification, energy production or cell structure maintenance. | [76] |
| Brachionus calyciflorus and Daphnia pulex | 0.42, 0.83 or 1.66 mg/L ATX-a for 24 h or cyanobacterial extracts containing ATX-a | Percentage of survivorship in acute toxicity bioassays | Pure ATX-a reduced the survivorship of D. pulex to 33% at 1.66 mg/L, whereas in B. calyciflorus did not produce effects. Cyanobacterial extracts containing mixtures of different cyanotoxins and other cyanobacterial metabolites were more toxic than pure toxins at lower concentrations. | [77] |
| Daphnia magna | Concentrations ranged from 0.5 to 50 µg/mL ATX-a for 24 h | Swimming response Measurement of oxygen consumption, heart rate and thoracic limb activity | Changes in swimming behavior were noted after treatment. A reduction in a concentration- and time-dependent manner of heart rate, oxygen consumption and thoracic limb activity was observed. | [78] |
| Female medaka fish (Oryzias latipes) | Single dose of 0.2–20 µg ATX-a by gavage | Behavioral study for 30 min Bioaccumulation of toxin in gut, liver and muscle by UHPLC Analysis of liver metabolomes by LC–MS/MS Determination of LD50 and NOAEL | The higher dose without effects was 6.67 µg/g and the oral LD50 and LD100 were 11.5 µg/g and 20 µg/g, respectively. Moreover, fish showed effects such as abnormal swimming and musculature rigidity among others. The content of the toxin decreased rapidly in tissues: after 12 h, ATX-a could not be detected in the liver, or after 3 days in the gut and muscles. Analysis of metabolome suggested a complete recovery 24 h after treatment with a NOAEL dose of toxin. | [79] |
| Daphnia magna clones and newborns from treated D. magna clones | Exposure to 100% Tychonema bourrelyi containing ATX-a or 50% T. bourrelyi + 50% Scenedemus obliquus for 4 days by diet | Measurement of juvenile somatic growth rates Quantification of NAR gene expression by qPCR | Treatment with 100% T. bourrelyi decreased the somatic growth rate and increased NAR gene expression. In contrast, with 50% T. bourrelyi, only a clone showed an increase in NAR expression without changes in growth rate. Moreover, this exposure to mothers affected to their offspring, showing a higher growth rate. | [80] |
| Birds | ||||
| Mallard ducks (and other species, see fish and mammmals) | Oral or i.p. doses of lyophilized Anabaena flos-aquae NRC-44-1 | Clinical observations | Animals showed opisthotonus and muscular rigidity. | [69] |
| Chick, mallard duck and ring-necked pheasant (and other species, see fish and mammmals) | I.p. injection or oral doses of Anabaena flos-aquae NRC-44-1 containing ATX-a | Determination of LD90 | When administration was oral, ducks were the most sensitive bird to ATX-a (LD90 = 350 mg/kg), followed by pheasants (LD90 = 850 mg/kg). Intraperitoneally, pheasants needed 2 times more dose (LD90 = 120 mg/kg) than ducks and chicks. | [68] |
| Mammals | ||||
| Calves, rats and mice (and other species, see fish and birds) | Oral or i.p. doses of Anabaena flos-aquae NRC-44-1 | Clinical observations and determination of MLD | Death was produced by respiratory arrest because of neuromuscular depolarizing activity. Oral MLD of calves was estimated to be 6–8 times higher than that of the mouse i.p. MLD/kg. The time to produce the death was 4–5 min for mice, 7 min for calves and 14–16 min for rats. | [69] |
| Mouse and rat (and other species, see fish and birds) | I.p. injection or oral doses of Anabaena flos-aquae NRC-44-1 containing ATX-a | Determination of LD90 | The oral LD90 for mice and rats were 1800 and 1500 mg/kg, respectively. The i.p. LD90 was equal in both species used (LD90 = 60 mg/kg). | [68] |
| Male mice | Oral or i.p. doses of Anabaena flos-aquae NRC-44-1 containing ATX-a | Clinical observations and Determination of LDmin | Animals showed convulsions and tremors. The LDmin obtained were 80 mg/kg i.p. and 800 mg/kg orally. | [81] |
| Calves | Administration of one or sequential doses of Anabaena flos-aquae NRC-44-1 by stomach tube | Analysis of blood samples and clinical observations | Loss of muscle coordination and muscle fasciculations were produced. Oral MLD was estimated in 420 mg/kg. | [82] |
| Female Sprague Dawley rats and pregnant Golden hamsters (Cricetus auratus) | Rats were exposed orally to 0.51 or 5.1 µg/mL ATX-a in drinking water for 7 weeks, or to 0.016 mg ATX-a daily i.p. doses for 21 days Hamsters received three i.p. doses of ATX-a at 0.125 or 0.2 mg/kg bw on gestation days 8–11 or 12–14 | Gross and microscopic analysis and measurement of enzymatic activities of AP, GPT, GGTP, CE | No adverse effects were seen in rats. Treatment of pregnant hamsters did not cause any malformations but caused stunting at all doses and periods compared with controls in 10–20% of fetuses. No maternal toxicity was observed. | [83] |
| NMRI-strain female mice | i.p. injections of 2.5–5 mg cyanobacteria blooms containing ATX-a | Determination of toxicity by mouse bioassay | The toxicity was different depending on the bloom sample. Anabaena species were present in all neurotoxic samples except one, in which Oscillatoria was the dominant species. The MLD obtained ranged from 50 to 500 mg/kg. | [4] |
| Male Sprague-Dawley rats | Intracerebroventricular or i.v. injections doses of 10, 30, 100 or 300 µg/kg ATX-a | Measurement of cardiac output by thermodilution technique Measurement of organ blood flow by Doppler technique Determination of catecholamines levels by UHPLC | The higher doses of toxin administered i.v. and intracerebroventricular produced a transient increase in cardiac output and vasoconstriction in the renal and mesenteric blood vessels. In addition, plasma epinephrine levels were increased two-fold with the dose of 100 µg/kg ATX-a. These effects were attenuated after chlorisondamine administration, a ganglion blocker. | [84] |
| Male Balb C mice and male Sprague Dawley rats | Mice were treated i.p. injection of 0.4–0.7 mL (+)ATX-a or (±)ATX-a; i.p. injections 1–73 mg/kg of (−)ATX-a Rats received 50–800 µg/kg of (+)ATX-a by i.v. injection | Behavioral study and measurement of ECAP | The LD50 for (+)ATX-a and (±)ATX-a were 386 µg/kg and 913 µg/kg, respectively. No deaths were observed with (−)ATX-a. The ED50 for depression of the ECAP was 47 mg/kg and the effects were dose-dependent. | [85] |
| Male Swiss Webster ND-4 mice | Daily single dose for 4 days or four doses in a day of pure (+)ATX-a or ATX-a derived from two different cyanobacterial extracts administered orally or i.p. | Determination of LD50 | More levels of toxin were necessary to produce death by oral route. The LD50 obtained was similar for all treatments when the administration was i.p. (0.23–0.28 mg/kg ATX-a). However, extract from Anabaena flos-aquae NCR-44-1 was 2-fold more potent (6.3–7.1 mg/kg) by oral route than pure toxin (15.4–17 mg/kg). | [28] |
| Male Sprague Dawley rats | Single i.v. dose ranging from 1 to 500 µg/kg of (+)ATX-a or (±)ATX-a | Measurement of blood pressure, heart rate, blood gases, pH and mortality | Lower doses of (+)ATX-a were necessary to produce the adverse effects. Nevertheless, both produced an increase in blood pressure and a decrease in heart rate, dose-dependent. In addition, hypoxemia, hypercapnia and acidosis were observed. LD50 for (+)ATX-a was ≈85 µg/kg and for (±)ATX-a was ≈400 µg/kg. | [86] |
| Male hooded rats | Subcutaneous injections of 10–200 µg/kg (+)ATX-a | Assessment of locomotor activity for 30 or 60 min | Reduction in locomotor activity either in nicotine-tolerant and non-tolerant rats. | [87] |
| Mouse | ATX-a was administered by gastric intubation, inhalation or i.p. injection ATX-a + MC-LR by intranasal route | Determination of LD50 | I.p. injection was the most sensitive administration route (LD50 = 375 µg/kg), followed by intranasal route (LD50 = 2000 µg/kg) and gastric intubation (LD50 = >5000 µg/kg). When ATX-a was administered together with MC-LR (31.3 µg/kg) by intranasal route, the LD50 decreased approximately 4-fold, at 500 µg/kg. | [29] |
| Crl:CD-1(ICR)BR mice | Single i.v. injection of 10–100 µg/kg (+) ATX-a or gavage doses of 0.098–15 mg/kg (+)ATX-a per day for 28 days Pregnant female mice were dosed by gavage 2.46 mg/kg (+)ATX-a daily between days 6–15 of pregnancy | Behavioral evaluation, assessment of locomotor activity and clinical observations | Animals showed salivation, hyperactivity and an increase in respiration after a single dose of toxin. The highest dose (100 µg/kg) produced the death in all treated mice. The NOAEL obtained in repeated doses was 0.098 mg/kg ATX-a. No adverse effects were observed in pregnant animals or their offspring. The NOAEL for teratogenicity was established at 2.46 mg/kg b.w. | [50] |
| Time-pregnant and non-pregnant CD-1 mice | I.p. dosages ATX-a ranged from 10 to 400 µg/kg in pregnant mice in a dose-finding assay Animals were treated with either 125 or 200 µg/kg for 5 days, on either GD 8–12 or GD 13–17 Mice received either 0, 500 or 1000 µg/kg of MC-LR by gavage and 50 min later, they received either 0, 500, 1000 or 2500 µg/kg ATX-a by gavage Mammalian embryos were exposed to 0.02, 0.2, 2.0 and 5.1 µg/mL ATX-a. | Evaluation for behavioral and physical alterations Analysis of morphogenesis by observation. | Adverse effects included difficult breathing, convulsions or altered gait in pregnant mice. At 200 µg/kg ATX-a, a reduced motor activity was observed and at the highest doses (300 and 400 µg/kg toxin), a 100% of mortality occurred. Nevertheless, no significant postnatal effects were observed in pups from any treatment group. No deaths were observed at any of the dose groups treated with MC-LR and ATX-a. Mammalian embryos exposed to 2.0 and 5.1 µg/mL showed perturbations in mouse yolk sac vasculature. | [73] |
| Female Sprague Dawley rats | Administration of 1, 2, 3.5 or 7 mM ATX-a by microdialysis probe (~281.3, 562.6, 984.55 or 1969.1 µg/mL) Toxin also was administered after exposure to different nicotinic or muscarinic receptors antagonists (MEC, MLA, atropine, α-bgt) | Determination of dopamine and metabolites by HPLC | An increase in striatal dopamine levels was produced in a dose-dependent way. There were not changes on release of dopamine metabolites. The combined used of ATX-a and different drugs indicated that ATX-a acts through nicotinic receptors. These results also support further in vivo evidence that α/β and α7 * nicotinic AChRs are involved in the striatal dopamine release induced by ATX-a. | [88] |
| Male Long Evans rats | Subcutaneous injections administered once a week for 4 weeks of 0.075–0.225 mg/kg (+)ATX-a or 0.20–0.95 mg/kg (±)ATX-a | Motor activity testing during 30 min sessions | Both forms, (+)ATX-a and (±)ATX-a, produced a reduction in locomotor activity horizontally and vertically after the first administration of the toxin. Weekly treatment did not change the effectiveness of the toxin. However, higher doses of racemic toxin were necessary to produce the acute effects. Neither form of toxin induced tolerance. | [89] |
| Male Long Evans rats | Four weekly subcutaneous injections of ATX-a doses ranged from 0.05 to 0.2 mg/kg | Behavioral study in trained rats | The toxin produced a dose-dependent reduction in response and reinforcement rates with the first administration. Tolerance was seen in behavioral responses after repeated administration with most doses, except for the highest dose (0.2 mg/kg ATX-a). | [90] |
| Male mice | Daily administration of 50, 100 or 150 µg/kg ATX-a by i.p. injection for seven days | Sperm counts and histopathological examinations on the testes | Dose-dependent reductions in epididymis weights and sperm count in all treatment groups. In addition, histopathological changes were observed, such as loosening of germ cells or degenerations in seminiferous tubules. | [91] |
| Female Sprague Dawley rats | 3.5 mM ATX-a (~984.55 µg/mL) was administered by microdialysis probe into the striatum Toxin also was administered together with MLA | Measurement of amino acids content by HPLC | Toxin increased levels of extracellular glutamate, GABA, taurine and dopamine. The combined used of ATX-a and MLA indicated that glutamate release depended on the activation of α7 nicotinic receptors. | [92] |
| Female Swiss albino mice | Doses of ATX-a by gavage, i.p. injection or feeding | Determination of LD50 using OECD 425 guideline | Mice were more sensitive to i.p. injection exposure. LD50 obtained was 0.231 mg/kg for i.p. injection, 10.6 mg/kg for gavage and 25 mg/kg for feeding. | [93] |
3.1. Aquatic Organisms
3.2. Birds
3.3. Mammals
3.4. Plants
4. Conclusions
5. Material and Methods
5.1. The Information Sources and Search Strategy
5.2. Eligibility and Exclusion Criteria
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kimura, Y.; Ogawa, K.; Suzuki, M.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W. A new procedure for the analysis and purification of naturally occurring anatoxin-a from the blue-green alga Anabaena flos-aquae. Toxicon 1989, 27, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Selwood, A.I.; Holland, P.T.; Wood, S.A.; Smith, K.F.; McNabb, P.S. Production of anatoxin-a and a novel biosynthetic precursor by the cyanobacterium Aphanizomenon issatschenkoi. Environ. Sci. Technol. 2007, 41, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Himberg, K.; Luukkainen, R.; Niemelä, S.I.; Poon, G.K.; Codd, G.A. Preliminary characterization of neurotoxic cyanobacteria blooms and strains from Finland. Toxicol. Assess 1989, 4, 339–352. [Google Scholar] [CrossRef]
- Park, H.D.; Watanabe, M.F.; Harda, K.; Nagai, H.; Suzuki, M.; Watanabe, M.; Hayashi, H. Hepatotoxin (microcystin) and neurotoxin (anatoxin-a) contained in natural blooms and strains of cyanobacteria from Japanese freshwaters. Nat. Toxins 1993, 1, 353–360. [Google Scholar] [CrossRef]
- Viaggiu, E.; Melchiorre, S.; Volpi, F.; Di Corcia, A.; Mancini, R.; Garibaldi, L.; Crichigno, G.; Bruno, M. Anatoxin-a toxin in the cyanobacterium Planktothrix rubescens from a fishing pond in northern Italy. Environ. Toxicol. 2004, 19, 191–197. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef]
- Namikoshi, M.; Murakami, T.; Watanabe, M.F.; Oda, T.; Yamada, J.; Tsujimura, S.; Nagai, H.; Oishi, S. Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 2003, 42, 533–538. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in three alkaline Rift Valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. J. Plankton Res. 2004, 26, 925–935. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Kim, B.; Kim, E.; Okino, T. Hepatotoxic microcystins and neurotoxic anatoxin-a in cyanobacterial blooms from Korean lakes. Environ. Toxicol. Water Qual. Int. J. 1998, 13, 225–234. [Google Scholar] [CrossRef]
- Carrasco, D.; Moreno, E.; Paniagua, T.; Hoyos, C.D.; Wormer, L.; Sanchis, D.; Cirés, S.; Martín-del-Pozo, D.; Codd, G.A.; Quesada, A. Anatoxin-a occurrence and potential cyanobacterial anatoxin-a producers in Spanish reservoirs 1. J. Phycol. 2007, 43, 1120–1125. [Google Scholar] [CrossRef]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.C.; Humbert, J.F.; Guette, C.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef]
- Merel, S.; Villarín, M.C.; Chung, K.; Snyder, S. Spatial and thematic distribution of research on cyanotoxins. Toxicon 2013, 76, 118–131. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Cyanobacterial Toxins: Anatoxin-a and Analogues; Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Testai, E. Cyanobacterial toxins. In Toxic Cyanobacteria in Water a Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 72–94. [Google Scholar]
- Spivak, C.E.; Witkop, B.; Albuquerque, E.X. Anatoxin-a: A novel, potent agonist at the nicotinic receptor. Mol. Pharmacol. 1980, 18, 384–394. [Google Scholar]
- Aguilera, A.; Haakonsson, S.; Martin, M.V.; Salerno, G.L.; Echenique, R.O. Bloom-forming cyanobacteria and cyanotoxins in Argentina: A growing health and environmental concern. Limnologica 2018, 69, 103–114. [Google Scholar] [CrossRef]
- Osswald, J.; Azevedo, J.; Vasconcelos, V.; Guilhermino, L. Experimental determination of the bioconcentration factors for anatoxin-a in juvenile rainbow trout (Oncorhynchus mykiss). Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 77–86. [Google Scholar]
- Pawlik-Skowrońska, B.; Toporowska, M.; Rechulicz, J. Simultaneous accumulation of anatoxin-a and microcystins in three fish species indigenous to lakes affected by cyanobacterial blooms. Oceanol. Hydrobio. Stud. 2012, 41, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toporowska, M.; Pawlik-Skowronska, B.; Kalinowska, R. Accumulation and effects of cyanobacterial microcystins and anatoxin-a on benthic larvae of Chironomus spp. (Diptera: Chironomidae). Eur. J. Entomol. 2014, 111, 83–90. [Google Scholar] [CrossRef]
- Rellán, S.; Osswald, J.; Saker, M.; Gago-Martinez, A.; Vasconcelos, V. First detection of anatoxin-a in human and animal dietary supplements containing cyanobacteria. Food Chem. Toxicol. 2009, 47, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.A.; Carmichael, W.W. The pharmacology of anatoxin-a(s), a neurotoxin produced by the freshwater cyanobacterium Anabaena flos-aquae NRC 525-17. Toxicon 1986, 24, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Biré, R.; Bertin, T.; Dom, I.; Hort, V.; Schmitt, C.; Diogène, J.; Lemée, R.; De Haro, L.; Nicolas, M. First evidence of the presence of anatoxin-a in sea figs associated with human food poisonings in France. Mar. Drugs 2020, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.K.; Krieger, R.I. Effect of route of exposure and repeated doses on the acute toxicity in mice of the cyanobacterial nicotinic alkaloid anatoxin-a. Toxicon 1991, 29, 134–138. [Google Scholar] [CrossRef]
- Fitzgeorge, R.B.; Clark, S.A.; Keevil, C.W. Routes of intoxication. In 1st International Symposium on Detection Methods for Cyanobacterial Toxins (Blue-Green Algal) Toxins; Codd, G.A., Jeffries, T.M., Kneevil, C.W., Potter, E., Eds.; Royal Society of Chemistry: Cambridge, UK, 1994. [Google Scholar]
- Osswald, J.; Carvalho, A.P.; Guimarães, L.; Guilhermino, L. Toxic effects of pure anatoxin-a on biomarkers of rainbow trout, Oncorhynchus mykiss. Toxicon 2013, 70, 162–169. [Google Scholar] [CrossRef]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identification of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef]
- James, K.J.; Sherlock, I.R.; Stack, M.A. Anatoxin-a in Irish freshwater and cyanobacteria, determined using a new fluorimetric liquid chromatographic method. Toxicon 1997, 35, 963–971. [Google Scholar] [CrossRef]
- Hamill, K.D. Toxicity in benthic freshwater cyanobacteria (blue-green algae): First observations in New Zealand. N. Z. J. Mar. Freshw. Res. 2001, 35, 1057–1059. [Google Scholar] [CrossRef]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of anatoxin-a poisoning in dogs from North America. J. Vet. Diagn. Investig. 2008, 20, 89–92. [Google Scholar] [CrossRef]
- Puschner, B.; Pratt, C.; Tor, E.R. Treatment and diagnosis of a dog with fulminant neurological deterioration due to anatoxin--a intoxication. J. Vet. Emerg. Crit. Care 2010, 20, 518–522. [Google Scholar] [CrossRef]
- Faassen, E.J.; Harkema, L.; Begeman, L.; Lurling, M. First report of (homo)anatoxin-a and dog neurotoxicosis after ingestion of benthic cyanobacteria in The Netherlands. Toxicon 2012, 60, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Fastner, J.; Beulker, C.; Geiser, B.; Hoffmann, A.; Kröger, R.; Teske, K.; Hoppe, J.; Mundhenk, L.; Neurath, H.; Sagebiel, D.; et al. Fatal neurotoxicosis in dogs associated with Tychoplanktic, anatoxin-a producing Tychonema sp. in Mesotrophic Lake Tegel, Berlin. Toxins 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabart, M.; Crenn, K.; Perrière, F.; Abila, A.; Leremboure, M.; Colombet, J.; Jousse, C.; Latour, D. Co-occurrence of microcystin and anatoxin-a in the freshwater lake Aydat (France): Analytical and molecular approaches during a three-year survey. Harmful Algae 2015, 48, 12–20. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Kudela, R.M.; Power, M.E. Widespread anatoxin-a detection in benthic cyanobacterial mats throughout a river network. PLoS ONE 2018, 13, e0197669. [Google Scholar] [CrossRef] [Green Version]
- Casero, M.C.; Velázquez, D.; Medina-Cobo, M.; Quesada, A.; Cirés, S. Unmasking the identity of toxigenic cyanobacteria driving a multi-toxin bloom by high-throughput sequencing of cyanotoxins genes and 16S rRNA metabarcoding. Sci. Total Environ. 2019, 665, 367–378. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 19515. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Biggs, D.F.; Peterson, M.A. Pharmacology of anatoxin-a, produced by the freshwater cyanophyte Anabaena flos-aquae NRC-44-1. Toxicon 1979, 17, 229–236. [Google Scholar] [CrossRef]
- Aronstam, R.S.; Witkop, B. Anatoxin-a interactions with cholinergic synaptic molecules. Proc. Natl. Acad. Sci. USA 1981, 78, 4639–4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.L.; Allen, C.N.; Aronstam, R.S.; Rapoport, H.; Albuquerque, E.X. Molecular mechanisms of the potent and stereospecific nicotinic receptor agonist (+)-anatoxin-a. Mol. Pharmacol. 1986, 29, 250–257. [Google Scholar]
- Zhang, X.; Stjernlöf, P.; Adem, A.; Nordberg, A. Anatoxin-a a potent ligand for nicotinic cholinergic receptors in rat brain. Eur. J. Pharmacol. 1987, 135, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Stephens, M.; Wilkie, G.; Amar, M.; Lunt, G.G.; Whiting, P.; Gallagher, T.; Pereira, E.; Alkondon, M.; Albuquerque, E.X.; et al. (+)-Anatoxin-a is a potent agonist at neuronal nicotinic acetylcholine receptors. J. Neurochem. 1993, 60, 2308–2311. [Google Scholar] [CrossRef] [PubMed]
- Molloy, L.; Wonnacott, S.; Gallagher, T.; Brough, P.A.; Livett, B.G. Anatoxin-a is a potent agonist of the nicotinic acetylcholine receptor of bovine adrenal chromaffin cells. Eur. J. Pharmacol. 1995, 289, 447–453. [Google Scholar] [CrossRef]
- Soliakov, L.; Gallagher, T.; Wonnacott, S. Anatoxin-a-evoked [3H]dopamine release from rat striatal synaptosomes. Neuropharmacology 1995, 34, 1535–1541. [Google Scholar] [CrossRef]
- Fawell, J.K.; Mitchell, R.E.; Hill, R.E.; Everett, D.J. The toxicity of cyanobacterial toxins in the mouse: II anatoxin-a. Hum. Exp. Toxicol. 1999, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Puttfarcken, P.S.; Jabobs, I.; Faltynek, C. Assessment of nicotinic acetylcholine receptor-mediated release of [3H]-norepinephrine from rat brain slices using a new 96-well format assay. Neuropharmacology 2000, 39, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Rao Lakshmana, P.V.; Bhattacharya, R.; Gupta, N.; Parida, M.M.; Bhaskar, A.S.; Dubey, R. Involvement of caspase and reactive oxygen species in cyanobacterial toxin anatoxin-a-induced cytotoxicity and apoptosis in rat thymocytes and Vero cells. Arch. Toxicol. 2002, 76, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Teneva, I.; Mladenov, R.; Popov, N.; Dzhambazov, B. Cytotoxicity and apoptotic effects of microcystin-LR and anatoxin-a in mouse lymphocytes. Folia Biol. 2005, 51, 62–67. [Google Scholar]
- Sieroslawska, A.; Rymuszka, A. Evaluation of genotoxic potential of neurotoxin anatoxin-a with the use of umuC test. Neuro Endocrinol. Lett. 2010, 31, 16–20. [Google Scholar] [PubMed]
- Bownik, A.; Rymuszka, A.; Sierosławska, A.; Skowroński, T. Anatoxin-a induces apoptosis of leukocytes and decreases the proliferative ability of lymphocytes of common carp (Cyprinus carpio L.) in vitro. Pol. J. Vet. Sci. 2012, 15, 531–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rymuszka, A. Cytotoxic activity of the neurotoxin anatoxin-a on fish leukocytes in vitro and in vivo studies. Acta Vet. Brno 2012, 81, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Rymuszka, A.; Adaszek, Ł. Pro- and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress--an in vitro study. Fish Shellfish Immunol. 2012, 33, 382–388. [Google Scholar] [CrossRef]
- Sieroslawska, A. Assessment of the mutagenic potential of cyanobacterial extracts and pure cyanotoxins. Toxicon 2013, 74, 76–82. [Google Scholar] [CrossRef]
- Sierosławska, A.; Rymuszka, A. Experimental immunology Assessment of the potential genotoxic and proapoptotic impact of selected cyanotoxins on fish leukocytes. Cent. Eur. J. Immunol. 2013, 38, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at low doses induce apoptosis and inflammatory effects in murine brain cells: Potential implications for neurodegenerative diseases. Toxicol. Rep. 2016, 3, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hernández, S.E.; Swift, S.; Singhal, N. Estrogenic activity of cylindrospermopsin and anatoxin-a and their oxidative products by FeIII-B*/H2O2. Water Res. 2018, 132, 309–319. [Google Scholar] [CrossRef]
- Chia, M.A.; Kramer, B.J.; Jankowiak, J.G.; Bittencourt-Oliveira, M.D.C.; Gobler, C.J. The individual and combined effects of the cyanotoxins, anatoxin-a and microcystin-LR, on the growth, toxin production, and nitrogen fixation of prokaryotic and eukaryotic algae. Toxins 2019, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Shen, L.; Ye, X.; Zhou, D.; He, Y.; Li, Y.; Ding, Y.; Zhu, W.; Ding, J.; Zhang, H. Neurotoxic anatoxin-a can also exert immunotoxicity by the induction of apoptosis on Carassius auratus lymphocytes in vitro when exposed to environmentally relevant concentrations. Front. Physiol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Adamski, M.; Zimolag, E.; Kaminski, A.; Drukała, J.; Bialczyk, J. Effects of cylindrospermopsin, its decomposition products, and anatoxin-a on human keratinocytes. Sci. Total Environ. 2021, 765, 142670. [Google Scholar] [CrossRef] [PubMed]
- Zegura, B.; Straser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef] [PubMed]
- Abramsson-Zetterberg, L.; Sundh, U.B.; Mattsson, R. Cyanobacterial extracts and microcystin-LR are inactive in the micronucleus assay in vivo and in vitro. Mutat. Res. 2010, 699, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Biggs, D.F. Muscle sensitivity differences in two avian species to anatoxin-a produced by the freshwater cyanophyte Anabaena flos-aquae NRC-44-1. Can. J. Zool. 1978, 56, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Biggs, D.F.; Gorham, P.R. Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science 1975, 187, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, J.; Sivonen, K.; Niemelä, S.I.; Huovinen, K. Detection of toxicity of cyanobacteria by Artemia salina bioassay. Environ. Toxicol. Water Qual. 1991, 6, 423–436. [Google Scholar] [CrossRef]
- Lahti, K.; Ahtiainen, J.; Rapala, J.; Sivonen, K.; Niemelä, S.I. Assessment of rapid bioassays for detecting cyanobacterial toxicity. Lett. Appl. Microbiol. 1995, 21, 109–114. [Google Scholar] [CrossRef]
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of cyanobacterial toxins and aqueous crude extracts of cyanobacteria on the development of fish and amphibians. Environ. Toxicol. Int. J. 1999, 14, 77–88. [Google Scholar] [CrossRef]
- Rogers, E.H.; Hunter, E.S.; Moser, V.C.; Phillips, P.M.; Herkovits, J.; Muñoz, L.; Hall, L.L.; Chernoff, N. Potential developmental toxicity of anatoxin-a, a cyanobacterial toxin. J. Appl. Toxicol. 2005, 25, 527–534. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Carvalho, A.P.; Gago, A.; Vasconcelos, V. Acute effects of an anatoxin-a producing Cyanobacterium on juvenile fish-Cyprinus carpio L. Toxicon 2007, 49, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Osswald, J.; Carvalho, A.P.; Claro, J.; Vasconcelos, V. Effects of cyanobacterial extracts containing anatoxin-a and of pure anatoxin-a on early developmental stages of carp. Ecotoxicol. Environ. Saf. 2009, 72, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.; Gutiérrez-Praena, D.; Osório, H.; Vasconcelos, V.; Carvalho, A.P.; Campos, A. Proteomic analysis of anatoxin-a acute toxicity in zebrafish reveals gender specific responses and additional mechanisms of cell stress. Ecotoxicol. Environ. Saf. 2015, 120, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowrońska, B.; Toporowska, M.; Mazur-Marzec, H. Effects of secondary metabolites produced by different cyanobacterial populations on the freshwater zooplankters Brachionus calyciflorus and Daphnia pulex. Environ. Sci. Pollut. Res. Int. 2019, 26, 11793–11804. [Google Scholar] [CrossRef] [PubMed]
- Bownik, A.; Pawlik-Skowrońska, B. Early indicators of behavioral and physiological disturbances in Daphnia magna (Cladocera) induced by cyanobacterial neurotoxin anatoxin-a. Sci. Total Environ. 2019, 695, 133913. [Google Scholar] [CrossRef]
- Colas, S.; Duval, C.; Marie, B. Toxicity, transfer and depuration of anatoxin-a (cyanobacterial neurotoxin) in medaka fish exposed by single-dose gavage. Aquat. Toxicol. 2020, 222, 105422. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Martin-Creuzburg, D. Daphnia’s adaptive molecular responses to the cyanobacterial neurotoxin anatoxin-α are maternally transferred. Toxins 2021, 13, 326. [Google Scholar] [CrossRef]
- Devlin, J.P.; Edwards, O.E.; Gorham, P.R.; Hunter, N.R.; Pike, R.K.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Gorham, P.R.; Biggs, D.F. Two laboratory case studies on the oral toxicity to calves of the freshwater cyanophyte (blue-green alga) Anabaena flos-aquae NRC-44-1. Can. Vet. J. 1977, 18, 71–75. [Google Scholar]
- Astrachan, N.B.; Archer, B.G.; Hilbelink, D.R. Evaluation of the subacute toxicity and teratogenicity of anatoxin-a. Toxicon 1980, 18, 684–688. [Google Scholar] [CrossRef]
- Sirén, A.L.; Feuerstein, G. Cardiovascular effects of anatoxin-A in the conscious rat. Toxicol. Appl. Pharmacol. 1990, 102, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, W.M.; Schaeffer, D.J.; Beasley, V.R. Electromyographic assessment of the neuromuscular blockade produced in vivo by anatoxin-a in the rat. Toxicon 1991, 29, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, O.M.; Sirén, A.L. Cardio-respiratory changes and mortality in the conscious rat induced by (+)- and (+/−)-anatoxin-a. Toxicon 1992, 30, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Stolerman, I.P.; Albuquerque, E.X.; Garcha, H.S. Behavioural effects of anatoxin, a potent nicotinic agonist, in rats. Neuropharmacology 1992, 31, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Durán, R.; Vidal, L.; Faro, L.R.; Alfonso, M. In vivo effects of the anatoxin-a on striatal dopamine release. Neurochem. Res. 2006, 31, 491–501. [Google Scholar] [CrossRef]
- MacPhail, R.C.; Farmer, J.D.; Jarema, K.A. Effects of acute and weekly episodic exposures to anatoxin-a on the motor activity of rats: Comparison with nicotine. Toxicology 2007, 234, 83–89. [Google Scholar] [CrossRef]
- Jarema, K.A.; Poling, A.; MacPhail, R.C. Effects of weekly exposure to anatoxin-a and nicotine on operant performance of rats. Neurotoxicol. Teratol. 2008, 30, 220–227. [Google Scholar] [CrossRef]
- Yavasoglu, A.; Karaaslan, M.A.; Uyanikgil, Y.; Sayim, F.; Ates, U.; Yavasoglu, N.U. Toxic effects of anatoxin-a on testes and sperm counts of male mice. Exp. Toxicol. Pathol. 2008, 60, 391–396. [Google Scholar] [CrossRef]
- Campos, F.; Alfonso, M.; Durán, R. In vivo modulation of alpha7 nicotinic receptors on striatal glutamate release induced by anatoxin-A. Neurochem. Int. 2010, 56, 850–855. [Google Scholar] [CrossRef]
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P.; et al. Acute toxicity of dihydroanatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a. Chemosphere 2021, 263, 127937. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Krause, E.; Kotut, K.; Pütz, S.; Wiegand, C.; Pflugmacher, S.; Codd, G.A. Analysis of the cyanotoxins anatoxin-a and microcystins in Lesser Flamingo feathers. Toxicol. Environ. Chem. 2006, 88, 159–167. [Google Scholar] [CrossRef]
- Nowruzi, B.; Blanco, S.; Nejadsattari, T. Chemical and molecular evidences for the poisoning of a duck by anatoxin-a, nodularin and cryptophycin at the coast of lake Shoormast (Mazandaran province, Iran). Algologia 2018, 28, 409–427. [Google Scholar] [CrossRef]
- Stepanova, N.; Nikitin, O. Cyanotoxins as a possible cause of fish and waterfowl death in the Kazanka River (Russia). Sect. Ecol. Environ. Prot. 2018, 18, 229–236. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Pflugmacher, S.; James, K.J.; Furey, A. Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat. Toxicol. 2004, 68, 185–192. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Jung, K.; Lundvall, L.; Neumann, S.; Peuthert, A. Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ. Toxicol. Chem. 2006, 25, 2381–2387. [Google Scholar] [CrossRef]
- Ha, M.H.; Pflugmacher, S. Phytotoxic effects of the cyanobacterial neurotoxin anatoxin-a: Morphological, physiological and biochemical responses in aquatic macrophyte, Ceratophyllum demersum. Toxicon 2013, 70, 1–8. [Google Scholar] [CrossRef]
- Ha, M.H.; Pflugmacher, S. Time-dependent alterations in growth, photosynthetic pigments and enzymatic defense systems of submerged Ceratophyllum demersum during exposure to the cyanobacterial neurotoxin anatoxin-a. Aquat. Toxicol. 2013, 15, 26–34. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, C.; Luo, X.; Zhang, H.; Zhang, J.; Zheng, Z. Combined toxic effects of anatoxin-a and microcystin-LR on submerged macrophytes and biofilms. J. Hazard. Mater. 2020, 389, 122053. [Google Scholar] [CrossRef]





| Experimental Models | Experimental Conditions | Assays Performed | Main Results | References |
|---|---|---|---|---|
| Isolated muscle preparations of Rana pipiens frog-rectus abdominus, chick biventer cervicis, rat phrenic nerve hemidiaphragm, cat sciatic nerve-anterior tibialis and guinea pig ileum | 0.5 to 10 µM of (~0.0826–1.65 µg/mL) extracted or synthetic ATX-a. | Muscle response/maximal response. | Extracted and synthetic ATX-a had similar pharmacological properties. ATX-a had a potency greater than acetylcholine or carbachol on striated muscle. Tetrodotoxin had no significant effect on ATX-a responses. ATX-a had ganglionic stimulating effects on the smooth muscle of guinea pig ileum. ATX-a showed less potent but qualitatively similar action to decamethonium. | [43] |
| Electric organs of Torpedo ocellata and neural membranes of male Wistar rats | Log (−8 to −5.5) M of ATX-a for 5 min (~0.0017–0.522 µg/mL) | Binding assays: Measurement of the radioactivity associated with the tissue trapped on the filters | ATX-a stimulated the “ion channel blockers” such as [3H]perhydrohistrionicotoxin, [3H]phencyclidine and [3H]phencyclidine methiodide with a range of EC50 from 0.14 to 0.28 µM for these effects. EC50 of ATX-a for inhibition of 3-[3H]quinuclidinyl benzilate binding was between 10 and 20 µM, and a low affinity of this toxin for the muscarinic acetylcholine receptors of rat brain was shown. | [44] |
| Isolated muscle preparations of frog-rectus abdominus and hindfoot of the Rana pipiens Electric organs of Torpedo californica | Log (−8 to −4) M (~0.0017–16.52 µg/mL) of (+)ATX-a or (−)ATX-a 10 µM (~1.65 µg/mL) of (+)ATX-a for 5 min for binding measure | Potency assay. Binding assays: Measurement of the radioactivity associated with the tissue trapped on the filters Electrophysiological techniques such as patch clamping | (+)ATX-a was a more potent agonist than ACh or carbamylcholine because of a higher affinity for the nicotinic acetylcholine receptor, whereas (−)ATX-a was less potent than carbamylcholine. At various concentrations the toxin activates the appearance of channels with the same conductances as ACh-induced channels but with a shorter lifetime. | [45] |
| Cortical brain rat tissue | Log (−10 to −4) M (~0.000017 to 16.52 µ/mL) of (+)ATX-a, (−)ATX-a or (±)ATX-a | [3H]ACh binding assay: measurement of the radioactivity associated with the tissue trapped on the filters. | (+)ATX-a (IC50 4.5 nM) was 160-fold more potent than the (−)ATX-a (IC50 750 nM) and 20-fold more potent than (±)ATX-a (IC50 30 nM) in inhibiting [3H]Ach binding: (+)ATX-a > (±)ATX-a > (−)ATX-a Hill coefficients of 0.56, 0.44 and 0.52 were obtained for (+)ATX-a, (±)ATX-a and (−)nicotine, respectively. | [46] |
| Rat hippocampal synaptosomes Mouse M10 cells (that expressed chicken α4β2 nAChR subunits) Xenopus oocytes (α7 nAChR) Fetal rat hippocampal neurons | Log (−9 to −3) M (~0.00017 to 165.237 µg/mL) of (+)ATX-a | Patch-clamp technique was used to record whole-cell currents from fetal rat hippocampal neurones cultured for 20–45 days Activation of the nAChR was measured as the stimulation of 86Rb+ influx into the cells by treatment with the agonist for 1 min Conventional dual-electrode voltage clamp for electrophysiological recording | The EC50 of (+)ATX-a in presynaptic nAChR, α4β2 nAChR, α7 nAChR and hippocampal neurones were 1.4 × 10−7, 4.8 × 10−8, 5.8 × 10−7 and 3.9 × 10−6, respectively. This toxin was between 3–50 times more potent than (−)-nicotine and 20 times more potent than acetylcholine. (+)ATX-a is the most efficacious nicotinic agonist. | [47] |
| Bovine adrenal chromaffin cells | ATX-a: 0.1–100 µM (~0.0165–16.52 µg/mL) for 5 min. | Release of catecholamines: High pressure liquid chromatography for catecholamines separation and detection | ATX-a was a potent agonist of the neuronal-type nicotinic receptor. It evoked higher secretion of noradrenaline and adrenaline than nicotine (EC50 1–2 µM vs. EC50 4–5 µM). Mecamylamine (at 10 µM for 5 min) inhibited the catecholamines secretion produced by ATX-a (at 5 µM for 5 min). At high concentrations of ATX-a (10 µM) the release of noradrenaline and adrenaline in the presence of 50 mM additional K+ was similar to that of ATX-a alone. | [48] |
| Synaptosomes of male Sprague Dawley rats | 0.01–100 µM ATX-a (~0.00165–16.52 µg/mL) by superfusion | Measurement of radioactivity each two minutes in a Packard scintillation spectrometer | [3H]dopamine was released in striatal synaptosomes in a concentration-dependent way after treatment with the toxin. The EC50 was 0.11 µM. This release was dependent of Ca2+. | [49] |
| In vitro organs bath preparations: Guinea-pig ileum Rat phrenic nerve diaphragm Chick biventer cervicis In vivo experiments: see Table 2 | ATX-a: 0.005–0.5 µg/mL | % response of tissue | ATX-a was 7-, 136- and 24-fold more potent as an agonist than nicotine in guinea-pig ileum, rat phrenic nerve diaphragm and chick biventer cervicis, respectively. The addition of the hexamethonium, a ganglion blocker, produced a parallel shift in the dose response curves for ATX-a and nicotine. Authors suggested a guideline value for ATX-a in drinking water of 1 µg/L. | [50] |
| Rat brain slices | Log (−10 to −3) M (~0.000017 to 165.237 µg/mL) of (+)ATX-a for 5 min | Concentration–response assays of nicotinic agonist-evoked release of [3H]-norepinephrine | ATX-a produced the concentration-dependent release of [3H]-NE in slices of hippocampus, thalamus and cortex. Concentration–response curve-revealed values of EC50 = 0.23, 0.13 and 0.15 µM in the hippocampus, thalamus and frontal cortex, respectively. Compared with other agonists, the rank order of potency was (±)-epibatidine >> (+)ATX-a > A85380 > DMPP = NIC = (−)-cytisine. | [51] |
| Rat thymocytes and African green monkey kidney cells (Vero). | ATX-containing cell free extracts from Anabena flos aquae (ACE) (10 to 50 µg/mL) or purified (+)ATX-a (1–10 µg/mL) for 15 min, 3 h, 6 h or 24 h of treatment depending on the assay | Cytotoxicity was measured by trypan blue exclusion assay, LDH leakage and MTT test Apoptosis by fluorescence staining and TUNEL assay Agarose gel electrophoresis for DNA fragmentation analysis Fluorescence for ROS quantification Fluorimetric assay for determination of caspase activity | ATX-a produced cytotoxicity, apoptosis and caspase-3 activation in both cell types. ACE and ATX-a induced ROS generation in rat thymocytes in a concentration- and time-dependent manner. ATX-induced apoptosis was mediated by caspase activation and ROS generation. | [52] |
| Spleen cells isolated from male BALB/c mice | 0.1 µg/mL ATX-a for 4, 24 or 48 h. | Cytotoxicity by MTT assay Apoptosis by flow cytometry | Time-dependent decrease in cell viability. Cytotoxic effects in a non-selective and non-specific manner. Both lymphocyte subpopulations (T and B cells) showed to be in late apoptotic or secondary necrotic phases after ATX-a exposure for 4 h. | [53] |
| Salmonella typhimurium TA 1535/pSK1002 | Toxin concentrations (ATX-a and ATX-a + MC-LR) were 0.25, 0.5, 1 and 2 μg/mL for 2 h. | umuC Easy CS Genotoxicity Assay kit to determinate the growth factor and β-galactosidase activity by spectrophotometry | In absence of S9 fraction, genotoxic effects and an increase in β-galactosidase activity at 0.5–2 µg/mL and 0.25–2 µg/mL ranges were observed for ATX-a and ATX-a + MC-LR mixture, respectively. In the presence of S9 fraction, no effects were detected in any samples. No effects in the growth factor in presence and absence of S9 fraction. | [54] |
| Lymphocytes of common carp | 0.01, 0.1, 1, 5 and 10 µg/mL of ATX-a for 24 h | Cytotoxicity by CellTiter-Glo® Luminescent Viability assay Determination of cell death type by cellular DNA fragmentation ELISA test kit Caspase-GloTM 3/7 Assay MTT Test for lymphocyte proliferative activity | A slight decrease in ATP levels and mild necrosis was observed only at the highest concentration tested. Cell apoptosis was observed after 24 h of exposure to 1, 5 and 10 µg/mL of ATX-a. Moreover, an early stage of apoptosis in these cells was confirmed by increased activity of effector caspases 3/7. The toxin also decreased the proliferation ability of lymphocytes in a concentration-dependent manner. | [55] |
| Immune cells from common carp | 0.01–1 µg/mL of ATX-a for 24 h | Cytotoxicity by bioluminescent assay, GSH assay and ROS production assay | Decreased ATP levels were not observed. ATX-a produced an increase in ROS at 0.01 and 0.025 µg/mL and a reduction in the respiratory burst activity at the highest concentration (1 µg/mL) in pronephros phagocytes. In blood phagocytes, the increase in ROS was observed at 0.05 µg/mL. | [56] |
| Head kidney leukocytes and blood leukocytes from common carp | Toxin concentrations (ATX-a and ATX-a extract) were 0.01 or 0.1 µg/mL for 4 h. | Gene expression of IL-1β, TNF-α, IL-10 and TGF-β cytokines by RT-PCR | Pure ATX-a dysregulated the expression of pro-inflammatory cytokines IL-1β and TNF-α more promptly than the anti-inflammatory cytokines TGF-β and IL-10. In general, pure ATX-a produced a significant increase in IL-1β, TNF-α and IL-10 expression in both cellular models. However, at 0.1 µg/mL, this toxin generated a significant decrease in TNF-α level. Contrary effects were observed after ATX-a extracts exposure in these cellular models. Thus, ATX-a extract produced a significant decrease in IL-1β and TNF-α expression levels. In addition, a significant increase in IL-10 level was observed after 0.1 µg/mL exposure in both cellular models. TGF-β was increased only in head kidney cells exposure to range of 0.01–0.1 µg/mL. | [57] |
| S. typhimurium TA98, TA100, TA1535, TA1537 and Escherichia coli WP2 uvrA and WP2 [pKM101] | Pure ATX-a in a range of 0.312–10 µg/mL. Mixture of ATX-a, CYN and MC-LR at 1 µg/mL. Different cyanobacterial extracts containing ATX-a, CYN and/or MC-LR. | Ames Test | Pure ATX-a and its mixture with CYN and MC-LR did not show mutagenic or cytotoxic effects. Some extracts containing cyanotoxins showed mutagenic effects in TA98 and TA100 bacterial strains. The results indicated that while tested cyanotoxins were not directly responsible for the observed mutagenicity of the extracts analysed, some synergistic interactions with other unidentified cyanobacterial-derived factors involved in the process were possible. | [58] |
| Common carp leukocytes | 0.5 µg/mL of ATX-a for 18 h | Comet assay | No genotoxic effects were observed in cells exposed to ATX-a. | [59] |
| Murine macrophage-like RAW264.7, microglial BV-2 and neuroblastoma N2a cell lines | 0.1 and 10 µM (~0.0281 and 2.813 µg/mL) of ATX-a Equimolar mixture of ATX-a, CYN and MC-LR at 0.001, 0.1 and 10 µM.(~0.000281, 0.0281 and 2.813 µg/mL) | Cellular viability by the MTT assay Apoptosis by measurement of the activation of caspases 3/7 Measurement of TNF-α protein by ELISA test | ATX-a did not reach LD50 levels of toxicity in any cell type, whereas equimolar mixture of toxins reached LD50 between 0.1–10 µM at different times (24, 48 or 72 h). The toxin mixture induced cell death of the N2a cells in a dose- and time-dependent manner. A measurement of 10 µM of toxins mixture induced almost a total death of RAW264.7 and BV-2 cells. Cytotoxicity: ATX-a < equimolar mixture of toxins in N2a cells < BV-2 cells < RAW264.7 cells ATX-a produced an induction of caspase activity, mainly when it is contained in the mixture of toxins. Moreover, N2a cells showed a higher sensitivity to ATX-a (alone or in the mixture) as compared to RAW264.7 and BV-2 cells. ATX-a significantly increased TNF-α secretion only in N2a cells. | [60] |
| Genetically modified strains of Saccharomyces cerevisiae (yeast cells) | 7.1 × 10−11 to 9.1 × 10−5 M (~0.00002 to 25.6 µg/mL) of ATX-a for 24 h | Flow cytometry for cell viability. YES assay for estrogenic response detection Q-Exactive Tandem Mass Spectrometry for detection the intermediate products of ATX-a | A significant reduction in viability in yeast cells was only observed after 4.5 × 10−5 M ATX-a exposure. ATX-a simulates endocrine-disrupting chemicals as it modulates the 17β-estradiol-induced estrogenic activity, resulting in non-monotonic dose responses. After the treatment with a high activity catalyst system (FeIII-B */H2O2), the ATX-a degradation products presented insignificant changes in its estrogenic activity. ATX-a was shown to induce estrogenic activity as agonist in the YES assay. | [61] |
| Microcystis spp., Anabaena variabilis and Selenastrum capricornutum | 25 µg/L of ATX-a 25 µg/L of ATX-a combined with 25 µg/L of MC-LR for 4 days | Flow cytometry to count cell density Fluorescence using Turner Designs TD-700 fluorometer to quantify chlorophyll-a concentration in the cultures ELISA kits for toxin quantification and measurement of antioxidant enzyme activities (SOD, POD, GST) GC for measurement of N2 fixation rates | ATX-a (alone or in mixture with MC-LR) produced a significant decrease in cell density and chlorophyll-a levels in Microcystis sp, and produced the opposite effects in S. capricornutum. In Anabaena, no changes were observed in these parameters after 4 days of exposure to the toxins. ATX-a increased antioxidant enzyme activities in Microcystis sp, which were unchanged or decreased in Anabaena UTEX B377 and S. capricornutum, respectively. ATX-a significantly inhibited nitrogen fixation by Anabaena UTEX B377. In general, the combined effects of these cyanotoxins were often more intense than their individual effects on some strains. | [62] |
| Lymphocytes of Carassius auratus | 0.01–10 mg/L of ATX-during 12 h | Analysis by electron microscopy, flow cytometry, electrophoresis and assay kits for antioxidant parameters | Vacuolation, swollen mitochondria and DNA fragmentations induced by ATX-a. Apoptosis in a concentration-dependent manner. Oxidative stress (↑ ROS and MDA; ↓ SOD, CAT, GR, GPx and GSH). | [63] |
| Human keratinocytes | 0.1, 1 or 10 µg/mL of ATX-a for 24, 48 or 72 h | WST-1 cell proliferation and crystal violet assay for proliferation Cytotoxicity detection kit (LDH) Scratch assay to describe the migratory activity of human keratinocytes | Cristal violet assay: in the proliferation of human keratinocytes, a toxic effect on the cells was only observed under the influence of the highest studied concentration. WST-1 assay: Toxic effects at 1 and 10 µg/mL. At 10 µg/mL, the decrease in cell proliferation was 60%, 81% and 84% after 24, 48 and 72 h, respectively. LDH: The toxicity of ATX-a was 24% after long incubation (48 h) at 10 µg/mL. No influence on keratinocyte migration was observed. | [64] |
| Experimental Model | Experimental Conditions | Assays Performed | Main Results | References |
|---|---|---|---|---|
| Lemma minor and Chladophora fracta | 5–25 µg/mL ATX-a for 4 days 0.1–20 µg/mL of ATX-a for 7 days for L. minor | Measurement of POD, CAT and GST activities and protein content Measurement of the macrophyte photosynthetic oxygen production | An increase in POD activity was observed in both organisms with the highest toxin concentration (25 µg/mL) after 4 days of treatment. Exposure of 7 days produced a rise in CAT and GST activities in L. minor at 5 and 20 µg/mL ATX-a. Moreover, these concentrations of ATX-a decreased oxygen production. | [97] |
| Alfalfa (Medicago sativa) | 5 µg/L ATX-a for 7 days | Morphological changes Oxidative stress parameters | Toxin produced a 27-fold inhibition on development of primary root of alfalfa compared to the control. Similarly, oxidative stress was produced. An increase in LPO and SOD, POD and GR activities was observed, as well as a decrease in CAT and GST activities. | [98] |
| Ceratophyllum demersum | 0.005–50 µg/L ATX-a for 24 h or 14 days | Oxidative stress parameters Analysis of chlorophyll and carotenoid contents by spectrometry | Concentrations greater than 0.5 µg/L led an inhibition of fresh weight. Toxin also decreased chlorophyl a content at 5 and 50 µg/L. H2O2 levels and GST, POD, SOD, GR, MDAR and APX activities were increased in a concentration-dependent manner. | [99] |
| Ceratophyllum demersum | 15 µg/L (±)ATX-a for 8 weeks | Oxidative stress parameters Analysis of chlorophyll contents by spectrometry Determination of growth parameters | The toxin produced oxidative stress. An increase in H2O2 levels and GST, POD, SOD, GR and APX activities were observed. Moreover, changes in chlorophyll contents were produced. Inhibition of fresh weight gain detected after 1 week exposure. | [100] |
| Vallisneria natans | 0.05–5 µg/L ATX-a or 0.05–5 µg/L MC-LR + ATX-a | Measurement of enzymatic biomarkers Determination of phytohormones by ELISA Analysis of biofilms | The toxin induced changes in oxidative stress biomarkers. An increase in CAT, POD and SOD activities and GSH content were observed. ATX-a also produced a rise in phytohormones and altered biofilms. A decrease in the biomass of plants was produced in all groups treated. Combined toxin treatment produced a reduction in SOD and POD activities compared with single toxin, showing an antagonistic effect. | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plata-Calzado, C.; Prieto, A.I.; Cameán, A.M.; Jos, A. Toxic Effects Produced by Anatoxin-a under Laboratory Conditions: A Review. Toxins 2022, 14, 861. https://doi.org/10.3390/toxins14120861
Plata-Calzado C, Prieto AI, Cameán AM, Jos A. Toxic Effects Produced by Anatoxin-a under Laboratory Conditions: A Review. Toxins. 2022; 14(12):861. https://doi.org/10.3390/toxins14120861
Chicago/Turabian StylePlata-Calzado, Cristina, Ana I. Prieto, Ana M. Cameán, and Angeles Jos. 2022. "Toxic Effects Produced by Anatoxin-a under Laboratory Conditions: A Review" Toxins 14, no. 12: 861. https://doi.org/10.3390/toxins14120861