FeOx-Modified Ultrafine Platinum Particles Supported on MgFe2O4 with High Catalytic Activity and Promising Stability toward Low-Temperature Oxidation of CO
Abstract
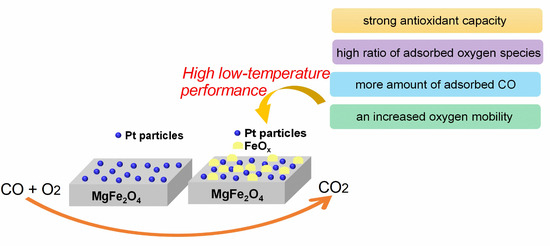
:1. Introduction
2. Results
2.1. Catalyst Performance
2.2. Catalyst Characterization
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Performance Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Tian, H.; Hua, S.; Zhu, C.; Gao, J.; Xue, Y.; Hao, J.; Wang, Y.; Zhou, J. A comprehensive emission inventory of multiple air pollutants from iron and steel industry in China: Temporal trends and spatial variation characteristics. Sci. Total Environ. 2016, 559, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, Y.; Li, X.; Xu, T.; Cen, W.; Li, B.; Zhu, T. Ultralow doping of Mn species into Pt catalyst enhances the CO oxidation performance in the presence of H2O and SO2. ACS Catal. 2023, 13, 14580–14597. [Google Scholar] [CrossRef]
- Waugh, K.C.; Butler, D.; Hayden, B.E.J.C.L. The mechanism of the poisoning of ammonia synthesis catalysts by oxygenates O2, CO and H2O: An in situ method for active surface determination. Catal. Lett. 1994, 24, 197–210. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.; Zhang, T. Recent advances in preferential oxidation of CO reaction over platinum group metal catalysts. ACS Catal. 2012, 2, 1165–1178. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Gao, R.; Liang, X.; Yu, Q.; Deng, Y.; Liu, J.; Peng, M.; Jiang, Z.; Li, S.; et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat. Nanotechnol. 2019, 14, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chi, X.; Li, L.; Yang, J.; Liu, S.; Lu, X.; Xiao, W.; Wang, L.; Luo, Z.; Yang, W.; et al. Elucidating the nature of the Cu (I) active site in CuO/TiO2 for excellent low-temperature CO oxidation. ACS Appl. Mater. Interfaces 2020, 12, 7091–7101. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, L.; Bajdich, M.; García-Melchor, M.; Li, H.; He, J.; Wilcox, J.; Wu, W.; Vojvodic, A.; Zheng, X. Enhancing catalytic CO oxidation over Co3O4 nanowires by substituting Co2+ with Cu2+. ACS Catal. 2015, 5, 4485–4491. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Henkelman, G. CO oxidation on the Pd (111) surface. ACS Catal. 2014, 4, 3435–3443. [Google Scholar] [CrossRef]
- Guan, H.; Lin, J.; Qiao, B.; Yang, X.; Li, L.; Miao, S.; Liu, J.; Wang, A.; Wang, X.; Zhang, T. Catalytically active Rh sub-nanoclusters on TiO2 for CO oxidation at cryogenic temperatures. Angew. Chem. Int. Ed. 2016, 55, 2820–2824. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gueddida, S.; Badawi, M.; Lebègue, S.; Giraudon, J.-M.; Dhainaut, J.; Royer, S.; Lamonier, J.-F. Unravelling the critical role of silanol in Pt/SiO2 for room temperature HCHO oxidation: An experimental and DFT study. Appl. Catal. B Environ. 2023, 331, 122672. [Google Scholar] [CrossRef]
- Xu, L.; Pan, Y.; Li, H.; Xu, R.; Sun, Z. Highly active and water-resistant lanthanum-doped platinum-cobalt oxide catalysts for CO oxidation. Appl. Catal. B Environ. 2023, 331, 122678. [Google Scholar] [CrossRef]
- Wang, X.-F.; Xu, L.-Y.; Wen, C.-H.; Li, D.-D.; Li, B.; Lu, J.-Q.; Yang, Q.-H.; Luo, M.-F.; Chen, J. WO3 boosted water tolerance of Pt nanoparticle on SO42−-ZrO2 for propane oxidation. Appl. Catal. B Environ. 2023, 338, 123000. [Google Scholar] [CrossRef]
- Lu, J.; Stair, P.C. Low-temperature ABC-type atomic layer deposition: Synthesis of highly uniform ultrafine supported metal nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Pellin, M.; Greeley, J.; Marshall, C.; Curtiss, L.; Ballentine, G.; Elam, J.; Catillon-Mucherie, S.; Redfern, P.; Mehmood, F.; et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Siani, A.; Alexeev, O.S.; Captain, B.; Lafaye, G.; Marécot, P.; Adams, R.D.; Amiridis, M.D. Synthesis of cluster-derived PtFe/SiO2 catalysts for the oxidation of CO. J. Catal. 2008, 255, 162–179. [Google Scholar] [CrossRef]
- Tomita, A.; Shimizu, K.-i.; Kato, K.; Tai, Y. Pt/Fe-containing alumina catalysts prepared and treated with water under moderate conditions exhibit low-temperature CO oxidation activity. Catal. Commun. 2012, 17, 194–199. [Google Scholar] [CrossRef]
- Song, S.; Wu, Y.; Ge, S.; Wang, L.; Wang, Y.; Guo, Y.; Zhan, W.; Guo, Y. A facile way to improve Pt atom efficiency for CO oxidation at low temperature: Modification by transition metal oxides. ACS Catal. 2019, 9, 6177–6187. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, P.; Cao, H.; Bals, S.; Heeres, H.J.; Pescarmona, P.P. Pt/ZrO2 prepared by atomic trapping: An efficient catalyst for the conversion of glycerol to lactic acid with concomitant transfer hydrogenation of cyclohexene. ACS Catal. 2019, 9, 9953–9963. [Google Scholar] [CrossRef]
- Meher, S.K.; Cargnello, M.; Troiani, H.; Montini, T.; Rao, G.R.; Fornasiero, P. Alcohol induced ultra-fine dispersion of Pt on tuned morphologies of CeO2 for CO oxidation. Appl. Catal. B Environ. 2013, 130, 121–131. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, Y.; Yang, X. Promoting effect of Pt on the activity and stability of Pd/MgFe2O4 for catalytic combustion of methane. J. Energy Inst. 2023, 110, 101341. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Li, X.; Liao, W.-M.; Jia, A.-P.; Wang, Y.-J.; Luo, M.-F.; Lu, J.-Q. Highly active Pt/BN catalysts for propane combustion: The roles of support and reactant-induced evolution of active sites. ACS Catal. 2019, 9, 1472–1481. [Google Scholar] [CrossRef]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal–support interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-P.; Chen, J.; Yu, H.-B.; Cen, B.-H.; Wang, W.-Y.; Luo, M.-F.; Lu, J.-Q. Insights into propane combustion over MoO3 promoted Pt/ZrO2 catalysts: The generation of Pt-MoO3 interface and its promotional role on catalytic activity. J. Catal. 2020, 391, 80–90. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Deng, J.; Yu, X.; Han, Z.; Zhang, K.; Dai, H. Alloying of gold with palladium: An effective strategy to improve catalytic stability and chlorine-tolerance of the 3DOM CeO2-supported catalysts in trichloroethylene combustion. Appl. Catal. B Environ. 2019, 257, 117879. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Chen, X.; Fan, S.; Zheng, Y. Engineering multicomponent metal-oxide units for efficient methane combustion over palladium-based catalysts. Catal. Sci. Technol. 2021, 11, 152–161. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt–Pd bimetallic nanoparticles anchored on uniform mesoporous MnO2 sphere as an advanced nanocatalyst for highly efficient toluene oxidation. Green Energy Environ. 2022, 7, 1349–1360. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Amal, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef]
- Zhu, M.-T.; Zhang, K.-F.; Du, W.-P.; Jia, A.-P.; Luo, M.-F.; Lu, J.-Q. Highly active and water tolerant Pt/MFe2O4 (M = Co and Ni) catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 2021, 619, 118142. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Kim, M.H.; Ebner, J.R.; Friedman, R.M.; Vannice, M.A. Dissociative N2O adsorption on supported Pt. J. Catal. 2001, 204, 348–357. [Google Scholar] [CrossRef]
- Meunier, F.C.; Cardenas, L.; Kaper, H.; Šmíd, B.; Vorokhta, M.; Grosjean, R.; Aubert, D.; Dembélé, K.; Lunkenbein, T. Synergy between metallic and oxidized Pt sites unravelled during room temperature CO oxidation on Pt/Ceria. Angew. Chem. Int. Ed. 2021, 60, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, W.; Wang, X.; Liu, C.; Lu, J.; Luo, M.; Chen, J. The effects of MoO3 impregnation order on the catalytic activity for propane combustion over Pt/ZrO2 catalysts: The crucial roles of Pt–MoO3 interfacial sites density. New J. Chem. 2021, 45, 14695–14702. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Zhang, G.; Qiu, W.; He, H.; Chen, G. High active platinum clusters on titanium dioxide supports toward carbon monoxide oxidation. Appl. Catal. B Environ. 2020, 266, 118629. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.-F.; Wang, W.-Y.; Liu, C.-F.; He, L.-C.; Luo, M.-F.; Chen, J. Identifying the surface active sites of FeOx-modified Pt/Nb2O5 catalysts in CO and propane oxidation. Appl. Catal. A Gen. 2023, 649, 118960. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, D.; Kim, B.-S.; Bae, J.; Shin, S.; Choe, C.; Han, J.W.; Lee, H. Controlling the oxidation state of Pt single atoms for maximizing catalytic activity. Angew. Chem. Int. Ed. 2020, 59, 20691–20696. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt particle size and valence state on the performance of Pt/TiO2 catalysts for CO oxidation at room temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]








| Samples | SBET (m2/g) | Pore Size a (nm) | Pore Volume (cm3/g) | Pt Loading (wt.%) | dSTEM (nm) | (Pt0) b % | (Oads) c % | (Pt0) d % | (Oads) e % |
|---|---|---|---|---|---|---|---|---|---|
| 3Pt/MgFe2O4 | 93.8 | 11.3 | 0.29 | 3.1 | 1.2 | 43.1 | 31.7 | 24.5 | 32.5 |
| 3FeOx-3Pt/MgFe2O4 | 77.9 | 11.5 | 0.25 | 2.9 | 1.3 | 49.0 | 37.4 | 31.7 | 40.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, F.; Shi, J. FeOx-Modified Ultrafine Platinum Particles Supported on MgFe2O4 with High Catalytic Activity and Promising Stability toward Low-Temperature Oxidation of CO. Molecules 2024, 29, 1027. https://doi.org/10.3390/molecules29051027
Wang C, Wang F, Shi J. FeOx-Modified Ultrafine Platinum Particles Supported on MgFe2O4 with High Catalytic Activity and Promising Stability toward Low-Temperature Oxidation of CO. Molecules. 2024; 29(5):1027. https://doi.org/10.3390/molecules29051027
Chicago/Turabian StyleWang, Chanchan, Fen Wang, and Jianjun Shi. 2024. "FeOx-Modified Ultrafine Platinum Particles Supported on MgFe2O4 with High Catalytic Activity and Promising Stability toward Low-Temperature Oxidation of CO" Molecules 29, no. 5: 1027. https://doi.org/10.3390/molecules29051027