A Comparative Study of the Semiconductor Behavior of Organic Thin Films: TCNQ-Doped Cobalt Phthalocyanine and Cobalt Octaethylporphyrin
Abstract
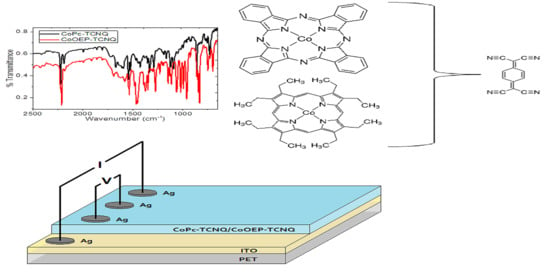
:1. Introduction
2. Results and Discussion
2.1. DFT Results and Discussion
2.2. Experimental Results and Discussion
3. DFT Calculations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sekitani, T.; Nakajima, H.; Maeda, H.; Fukushima, T.; Aida, T.; Hata, K.; Someya, T. Stretchable active-matrix organic light-emitting diode display using printable elastic conductors. Nat. Mater. 2009, 8, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef]
- Rao, M.C.; Shekhawat, M.S. A brief survey on basic properties of thin films for device application. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 576–582. [Google Scholar] [CrossRef]
- Alonso, M.I.; Garriga, M.; OssόJ, O.; Schreiber, F.; Barrena, E.; Dosch, H. Strong optical anisotropies of F[sub 16]CuPc thin films studied by spectroscopic ellipsometry. J. Chem. Phys. 2003, 119, 6335. [Google Scholar] [CrossRef] [Green Version]
- Melville, O.A.; Grant, T.M.; Lessard, B.H. Silicon phthalocyanines as N-type semiconductors in organic thin film transistors. J. Mater. Chem. C 2018, 6, 5482–5488. [Google Scholar] [CrossRef]
- Dang, M.-T.; Grant, T.M.; Yan, H.; Seferos, D.S.; Lessard, B.H.; Bender, T.P. Bis(tri-n-alkylsilyl oxide) silicon phthalocyanines: A start to establishing a structure property relationship as both ternary additives and non-fullerene electron acceptors in bulk heterojunction organic photovoltaic devices. J. Mater. Chem. A 2017, 5, 12168–12182. [Google Scholar] [CrossRef]
- Blochwitz, J.; Pfeiffer, M.; Fritz, T.; Leo, K. Low voltage organic light emitting diodes featuring doped phthalocyanine as hole transport material. Appl. Phys. Lett. 1998, 73, 729–731. [Google Scholar] [CrossRef]
- Bessho, T.; Zakeeruddin, S.M.; Yeh, C.-Y.; Diau, E.W.-G.; Grätzel, M. Highly Efficient Mesoscopic Dye-Sensitized Solar Cells Based on Donor-Acceptor-Substituted Porphyrins. Angew. Chem. Int. Ed. 2010, 49, 6646–6649. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.-H.; Jang, S.-R.; Humphry-Baker, R.; Gratzel, M.; Cid, J.-J.; Torres, T.; Nazeeruddin, M.K. Effect of Coadsorbent on the Photovoltaic Performance of Zinc Pthalocyanine-Sensitized Solar Cells. Langmuir 2008, 24, 5636–5640. [Google Scholar] [CrossRef] [PubMed]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, H.; Meng, Q.; Gong, X.; Hu, W. Organic photoresponse materials and devices. Chem. Soc. Rev. 2012, 41, 1754–1808. [Google Scholar] [CrossRef]
- Wong, R.C.H.; Lo, P.-C.; Ng, D.K. Stimuli responsive phthalocyanine-based fluorescent probes and photosensitizers. Co-ord. Chem. Rev. 2019, 379, 30–46. [Google Scholar] [CrossRef]
- Mrinalini, M.; Prasanthkumar, S. Recent Advances on Stimuli-Responsive Smart Materials and their Applications. ChemPlusChem 2019, 84, 1103–1121. [Google Scholar] [CrossRef] [Green Version]
- Mrinalini, M.; Krishna, J.V.S.; Krishna, N.V.; Kotha, V.; Panchakarla, L.S.; Prasanthkumar, S.; Giribabu, L. Photobleaching of Triphenylamine–Phthalocyanine Entails Mixed Valence-State Triggered Self-Assembled Nanospheres. J. Phys. Chem. C 2018, 122, 19946–19952. [Google Scholar] [CrossRef]
- Mrinalini, M.; Achary, B.S.P.; Ghosh, S.; Koteshwar, D.; Prasanthkumar, S.; Giribabu, L. Unveiling the Reversibility of Crystalline–Amorphous Nanostructures via Sonication-Induced Protonation. J. Phys. Chem. C 2018, 122, 10255–10260. [Google Scholar] [CrossRef]
- Li, X.; Lee, D.; Huang, J.-D.; Yoon, J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 9885–9890. [Google Scholar] [CrossRef]
- Mrinalini, M.; Pathak, S.S.; Achary, B.S.; Panchakarla, L.S.; Prasanthkumar, S.; Madoori, M.; Achary, S.P. Voltage Stimulated Anion Binding of Metalloporphyrin-induced Crystalline 2D Nanoflakes. Chem. Asian J. 2019, 14, 537–541. [Google Scholar] [CrossRef]
- Bi, H.; Palma, C.-A.; Gong, Y.; Hasch, P.; Elbing, M.; Mayor, M.; Reichert, J.; Barth, J.V. Voltage-Driven Conformational Switching with Distinct Raman Signature in a Single-Molecule Junction. J. Am. Chem. Soc. 2018, 140, 4835–4840. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, S.; Itoh, Y.; Tanaka, H.; Araoka, F.; Aida, T. Redox-Responsive Chiral Dopant for Quick Electrochemical Color Modulation of Cholesteric Liquid Crystal. J. Am. Chem. Soc. 2018, 140, 10946–10949. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, J.; Li, P.; Hod, I.; Moghadam, P.Z.; Kean, Z.S.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K.; Stoddart, J.F. A Redox-Active Bistable Molecular Switch Mounted inside a Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 14242–14245. [Google Scholar] [CrossRef] [PubMed]
- Güster, S.; Siebentritt, S.; Elbe, J.; Kreienhoop, L.; Tennigkeit, B.; Wöhrle, D.; Memming, R.; Meissner, D. Investigations of Porphyrins and Aromatic Tetracarboxylic Acid Diimides for use in Photovoltaics. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1992, 218, 117–122. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, L.; Zhou, X.; Li, L.; Li, Q. Bilayer- and bulk-heterojunction solar cells using liquid crystalline porphyrins as donors by solution processing. Appl. Phys. Lett. 2007, 91, 253505. [Google Scholar] [CrossRef] [Green Version]
- Huijser, A.; Savenije, T.J.; Shalav, A.; Siebbeles, L.D.A. An experimental study on the molecular organization and exciton diffusion in a bilayer of a porphyrin and poly(3-hexylthiophene). J. Appl. Phys. 2008, 104, 034505. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Li, H.; Song, Y.; Xu, W.; Hu, W.; Jiang, L.; Liu, Y.; Wang, X.; Zhu, D. In Situ Patterning of Organic Single-Crystalline Nanoribbons on a SiO2 Surface for the Fabrication of Various Architectures and High-Quality Transistors. Adv. Mater. 2006, 18, 3010–3014. [Google Scholar] [CrossRef]
- Tang, Q.; Tong, Y.; Hu, W.; Wan, Q.; Bjørnholm, T. Assembly of Nanoscale Organic Single-Crystal Cross-Wire Circuits. Adv. Mater. 2009, 21, 4234–4237. [Google Scholar] [CrossRef]
- Vivo, P.; Ojala, M.; Chukharev, V.; Efimov, A.; Lemmetyinen, H. Role of a phthalocyanine–fullerene dyad in multilayered organic solar cells. J. Photochem. Photobiol. A Chem. 2009, 203, 125–130. [Google Scholar] [CrossRef]
- Stübinger, T.; Brütting, W. Exciton diffusion and optical interference in organic donor–acceptor photovoltaic cells. J. Appl. Phys. 2001, 90, 3632–3641. [Google Scholar] [CrossRef]
- Harima, Y.; Furusho, S.; Okazaki, K.; Kunugi, Y.; Yamashita, K. Charge transport in vacuum-sublimed films of metal-free tetraphenylporphyrin and its relation to capacitance and photocurrent measurements. Thin Solid Films 1997, 300, 213–217. [Google Scholar] [CrossRef]
- Banerjee, S.; Parhi, A.P.; Iyer, S.S.K.; Kumar, S. Method of determining the exciton diffusion length using optical interference effect in Schottky diode. Appl. Phys. Lett. 2009, 94, 223303. [Google Scholar] [CrossRef]
- Yang, L.-G.; Chen, H.; Wang, M. Optimal film thickness for exciton diffusion length measurement by photocurrent response in organic heterostructures. Thin Solid Film. 2008, 516, 7701–7707. [Google Scholar] [CrossRef]
- Salzman, R.F.; Xue, J.; Rand, B.P.; Alexander, A.; Thompson, M.E.; Forrest, S.R. The effects of copper phthalocyanine purity on organic solar cell performance. Org. Electron. 2005, 6, 242–246. [Google Scholar] [CrossRef]
- Gould, R.D.C. electrical measurements on evaporated thin films of copper phthalocyanine. Thin Solid Films 1985, 125, 63–69. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Zhou, Z.; Xu, D.; Wang, Z.; Yang, Z.; Wei, H.; Kong, E.S.-W.; Yang, Z. Single-walled carbon nanotube/cobalt phthalocyanine derivative hybrid material: Preparation, characterization and its gas sensing properties. J. Mater. Chem. 2011, 21, 3779–3787. [Google Scholar] [CrossRef]
- Lindner, S.; Knupfer, M.; Friedrich, R.; Hahn, T.; Kortus, J. Hybrid States and Charge Transfer at a Phthalocyanine Heterojunction:MnPcδ+/F16CoPcδ−. Phys. Rev. Lett. 2012, 109, 027601. [Google Scholar] [CrossRef]
- Kobayashi, M.; Niwa, H.; Harada, Y.; Horiba, K.; Oshima, M.; Ofuchi, H.; Terakura, K.; Ikeda, T.; Koshigoe, Y.; Ozaki, J.-I.; et al. Role of residual transition-metal atoms in oxygen reduction reaction in cobalt phthalocyanine-based carbon cathode catalysts for polymer electrolyte fuel cell. J. Power Sources 2011, 196, 8346–8351. [Google Scholar] [CrossRef]
- Birnbaum, T.; Hahn, T.; Martin, C.; Kortus, J.; Fronk, M.; Lungwitz, F.; Zahn, D.R.T.; Salvan, G. Optical and magneto-optical properties of metal phthalocyanine and metal porphyrin thin films. J. Phys. Condens. Matter 2014, 26, 104201. [Google Scholar] [CrossRef] [PubMed]
- Hamui, L.; Sánchez, M.E.; Díaz-Ortega, N.; Salcedo, R. Comparative Study of Conduction Mechanisms in Disodium Phthalocyanine-Based Organic Diodes for Flexible Electronics. Molecules 2020, 25, 3687. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Molina, B.; Hernández-García, A.; Álvarez-Bada, J.R.; Salcedo, R. Growth and Characterization of TCNQ-Doped Ni(II)TAAB Thin Film as a New π-Conjugated Organic Semiconductor. Semiconductors 2020, 54, 441–449. [Google Scholar] [CrossRef]
- Walzer, K.; Maennig, B.; Pfeiffer, A.M.; Leo, K. Highly Efficient Organic Devices Based on Electrically Doped Transport Layers. Chem. Rev. 2007, 107, 1233–1271. [Google Scholar] [CrossRef] [PubMed]
- Kayunkid, N.; Rangkasikorn, A.; Saributr, C.; Nukeaw, J. Growth and characterizations of tin doped zinc-phthalocyanine prepared by thermal co-evaporation in high vacuum as a nanomaterial. Jpn. J. Appl. Phys. 2016, 55, 02BB12. [Google Scholar] [CrossRef]
- Abuelwafa, A.; El-Denglawey, A.; Dongol, M.; El-Nahass, M.; Soga, T. Influence of annealing temperature on structural and optical properties of nanocrystalline Platinum octaethylporphyrin (PtOEP) thin films. Opt. Mater. 2015, 49, 271–278. [Google Scholar] [CrossRef]
- Ogoshi, H.; Masai, N.; Yoshida, Z.-I.; Takemoto, J.; Nakamoto, K. The Infrared Spectra of Metallooctaethylporphyrins. Bull. Chem. Soc. Jpn. 1971, 44, 49–51. [Google Scholar] [CrossRef] [Green Version]
- Kincaid, J.R.; Urban, M.W.; Watanabe, T.; Nakamoto, K. Infrared spectra of matrix-isolated metal complexes of octaethylporphine. J. Phys. Chem. 1983, 87, 3096–3101. [Google Scholar] [CrossRef]
- Li, X.Y.; Czernuszewicz, R.S.; Kincaid, J.R.; Su, Y.O.; Spiro, T.G. Consistent porphyrin force field. 1. Normal-mode analysis for nickel porphine and nickel tetraphenylporphine from resonance Raman and infrared spectra and isotope shifts. J. Phys. Chem. 1990, 94, 31–47. [Google Scholar] [CrossRef]
- Scudiero, L.; Barlow, A.D.E.; Hipps, K.W. Scanning Tunneling Microscopy, Orbital-Mediated Tunneling Spectroscopy, and Ultraviolet Photoelectron Spectroscopy of Nickel(II) Octaethylporphyrin Deposited from Vapor. J. Phys. Chem. B 2002, 106, 996–1003. [Google Scholar] [CrossRef]
- Touka, N.; Benelmadjat, H.; Boudine, B.; Halimi, O.; Sebais, M. Copper phthalocyanine nanocrystals embedded into polymer host: Preparation and structural characterization. J. Assoc. Arab. Univ. Basic Appl. Sci. 2013, 13, 52–56. [Google Scholar] [CrossRef]
- El-Nahass, M.; Abd-El-Rahman, K.; Al-Ghamdi, A.; Asiri, A. Optical properties of thermally evaporated tin-phthalocyanine dichloride thin films, SnPcCl2. Phys. B Condens. Matter 2004, 344, 398–406. [Google Scholar] [CrossRef]
- El-Nahass, M.; El-Gohary, Z.; Soliman, H. Structural and optical studies of thermally evaporated CoPc thin films. Opt. Laser Technol. 2003, 35, 523–531. [Google Scholar] [CrossRef]
- Synthesis and Characterization of Tetrathiafulvalene (TTF) and 7,7,8,8-Tetracyanoquinodimethane (TCNQ) Compounds with PdX 2 (X=CI, NO 3 and Hexafluoroacetylacetonate). Bull. Korean Chem. Soc. 2002, 23, 1754–1758. [CrossRef] [Green Version]
- Medjanik, K.; Perkert, S.; Naghavi, S.; Rudloff, M.; Solovyeva, V.; Chercka, D.; Huth, M.; Nepijko, S.; Methfessel, T.; Felser, C.; et al. Formation of an intermolecular charge-transfer compound in UHV codeposited tetramethoxypyrene and tetracyanoquinodimethane. Phys. Rev. B 2010, 82, 245419. [Google Scholar] [CrossRef] [Green Version]
- Méndez, H.; Heimel, G.; Winkler, S.; Frisch, J.; Opitz, A.; Sauer, K.; Wegner, B.; Oehzelt, M.; Röthel, C.; Duhm, S.; et al. Charge-transfer crystallites as molecular electrical dopants. Nat. Commun. 2015, 6, 8560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szybowicz, M.; Bała, W.; Fabisiak, K.; Paprocki, K.; Drozdowski, M. The molecular structure ordering and orientation of the metallophthalocyanine CoPc, ZnPc, CuPc, and MgPc thin layers deposited on silicon substrate, as studied by micro-Raman spectroscopy. J. Mater. Sci. 2011, 46, 6589–6595. [Google Scholar] [CrossRef] [Green Version]
- Kayunkid, N.; Tammarugwattana, N.; Mano, K.; Rangkasikorn, A.; Nukeaw, J. Growth and characterizations of tin-doped nickel-phthalocyanine thin film prepared by thermal co-evaporation as a novel nanomaterial. Surf. Coat. Technol. 2016, 306, 101–105. [Google Scholar] [CrossRef]
- Gaffo, L.; Cordeiro, M.R.; Freitas, A.R.; Moreira, W.C.; Girotto, E.M.; Zucolotto, V. The effects of temperature on the molecular orientation of zinc phthalocyanine films. J. Mater. Sci. 2009, 45, 1366–1370. [Google Scholar] [CrossRef]
- Takenaka, T. Infrared and Raman Spectra of TCNQ and TCNQ-d4 Crystals. Bull. Inst. Chem. Res. Kyoto Univ. 1969, 47, 387–400. [Google Scholar]
- Gucciardi, P.G.; Trusso, S.; Vasi, C.S.; Patanè, S.; Allegrini, M. Nano-Raman imaging of Cu–TCNQ clusters in TCNQ thin films by scanning near-field optical microscopyPresented at the LANMAT 2001 Conference on the Interaction of Laser Radiation with Matter at Nanoscopic Scales: From Single Molecule Spectroscopy to Materials Processing, Venice, 3–6 October, 2001. Phys. Chem. Chem. Phys. 2002, 4, 2747–2753. [Google Scholar] [CrossRef]
- El-Nahass, M.; Ammar, A.; Farag, A.; Atta, A.; El-Zaidia, E. Effect of heat treatment on morphological, structural and optical properties of CoMTPP thin films. Solid State Sci. 2011, 13, 596–600. [Google Scholar] [CrossRef]
- Dongol, M.; El-Nahass, M.; El-Denglawey, A.; Elhady, A.; Abuelwafa, A. Optical Properties of Nano 5,10,15,20-Tetraphenyl-21H,23H-Prophyrin Nickel (II) Thin Films. Curr. Appl. Phys. 2012, 12, 1178–1184. [Google Scholar] [CrossRef]
- Ahmad, Z.; Sayyad, M.H.; Karimov, K.S. CuPc based organic-inorganic hetero-junction with Au electrodes. J. Semicond. 2010, 31, 074002. [Google Scholar] [CrossRef]
- Hassan, A.K.; Gouldt, R.D. The interpretation of current density–voltage and activation energy measurements on freshly prepared and heat-treated nickel phthalocyanine thin films. Int. J. Electron. 1993, 74, 59–65. [Google Scholar] [CrossRef]
- Gravano, S.; Hassan, A.K.; Gould, R.D. Effects of annealing on the trap distribution of cobalt phthalocyanine thin films. Int. J. Electron. 1991, 70, 477–484. [Google Scholar] [CrossRef]
- Gould, R. Structure and electrical conduction properties of phthalocyanine thin films. Co-ord. Chem. Rev. 1996, 156, 237–274. [Google Scholar] [CrossRef]
- Anthopoulos, T.; Shafai, T. SCLC Measurements in Nickel Phthalocyanine Thin Films. Phys. Status Solidi 2000, 181, 569–574. [Google Scholar] [CrossRef]
- Morales-Saavedra, O.G.; Sánchez-Vergara, M.; Rodríguez-Rosales, A.A.; Ortega-Martínez, R.; Ortiz-Rebollo, A.; Frontana-Uribe, B.; García-Montalvo, V. Synthesis and electrical, spectroscopic and nonlinear optical properties of cobalt molecular materials obtained from PcCo(CN)L (L=ethylenediamine, 1,4-diaminebutane, 1,12-diaminododecane and 2,6-diamineanthraquinone). Mater. Chem. Phys. 2010, 123, 776–785. [Google Scholar] [CrossRef]
- Salcedo, R.; Pérez-Manriquez, L.; Sánchez, M.E. Spectroscopic studies and density functional theory investigations of a cobalt phthalocyanine derivative. J. Mol. Struct. 2015, 1084, 165–171. [Google Scholar] [CrossRef]
- Bisquert, J.; Montero, J.M.; Bolink, H.J.; Barea, E.M.; Garcia-Belmonte, G. Thickness scaling of space-charge-limited currents in organic layers with field- or density-dependent mobility. Phys. Status Solidi 2006, 203, 3762–3767. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016; pp. 1–20. [Google Scholar]
- Bader, R.F.W. AIMPAC, Suite of Programs for the Theory of Atoms in Molecules, 1st ed.; McMaster University Press: Hamilton, ON, Canada, 1991. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| CoOEP-TCNQ Assignment | Powder as KBr Pellet (cm−1) | As-Deposited Films (cm−1) | CoPc-TCNQ Assignment | Powder as KBr Pellet (cm−1) | As-Deposited Films (cm−1) |
|---|---|---|---|---|---|
| δ (CH3) ethyl group | 1468 | 1466 | C=C stretching | 1611 | 1611 |
| Ring def. | 1230 | 1233 | C=C benzene stretching | 1476 | 1472 |
| Ring def. | 992 | 996 | In-plane pyrrole stretching | 1332 | 1337 |
| ρr (C2H5) | 924 | 925 | C-H bending | 1288, 1164, 1120 | 1289, 1163, 1120 |
| π (ring) | 755 | 752 | In plane C-H deformation | 754 | 754 |
| C≡N stretching bands of TCNQ | 2214, 1673 | 2215, 1671 | C≡N stretching bands of TCNQ | 2219, 1673 | 2221, 1676 |
| C=C-H bending of TCNQ | 1611 | 1615 | C=C-H bending of TCNQ | 1614 | 1615 |
| C-CN stretching of TCNQ | 1449 | 1451 | C-CN stretching of TCNQ | 1449 | 1449 |
| C=C ring stretching of TCNQ | 1209 | 1213 | C=C ring stretching of TCNQ | 1211 | 1207 |
| Device | μ (m2 V−1 s−1) | p0 (m−3) | P0 (J−1 m−3) | Nt(e) (m−3) |
|---|---|---|---|---|
| CoPc-TCNQ | 4.93 × 10−10 | 1.02 × 1025 | 9.92 × 1044 | 1.09 × 1025 |
Sample Availability: Samples of the compound are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Vergara, M.E.; Rios, C.; Jiménez-Sandoval, O.; Salcedo, R. A Comparative Study of the Semiconductor Behavior of Organic Thin Films: TCNQ-Doped Cobalt Phthalocyanine and Cobalt Octaethylporphyrin. Molecules 2020, 25, 5800. https://doi.org/10.3390/molecules25245800
Sánchez-Vergara ME, Rios C, Jiménez-Sandoval O, Salcedo R. A Comparative Study of the Semiconductor Behavior of Organic Thin Films: TCNQ-Doped Cobalt Phthalocyanine and Cobalt Octaethylporphyrin. Molecules. 2020; 25(24):5800. https://doi.org/10.3390/molecules25245800
Chicago/Turabian StyleSánchez-Vergara, María Elena, Citlalli Rios, Omar Jiménez-Sandoval, and Roberto Salcedo. 2020. "A Comparative Study of the Semiconductor Behavior of Organic Thin Films: TCNQ-Doped Cobalt Phthalocyanine and Cobalt Octaethylporphyrin" Molecules 25, no. 24: 5800. https://doi.org/10.3390/molecules25245800