Evaluation of Current Equine Influenza Vaccination Protocols Prior to Shipment, Guided by OIE Standards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Horses
2.3. Vaccines
2.4. Sample Collection
2.5. Serology
2.6. Statistical Analysis
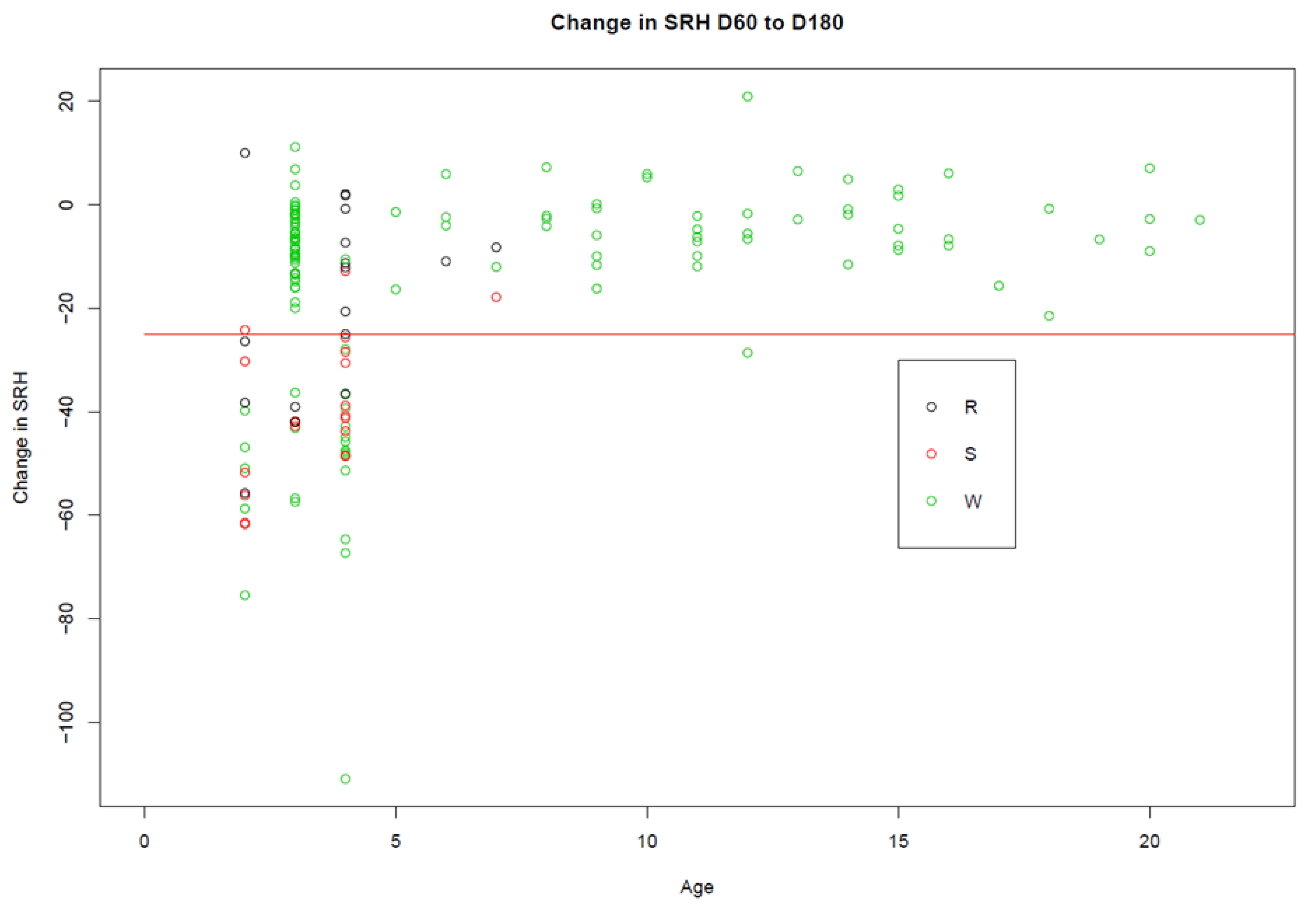
3. Results
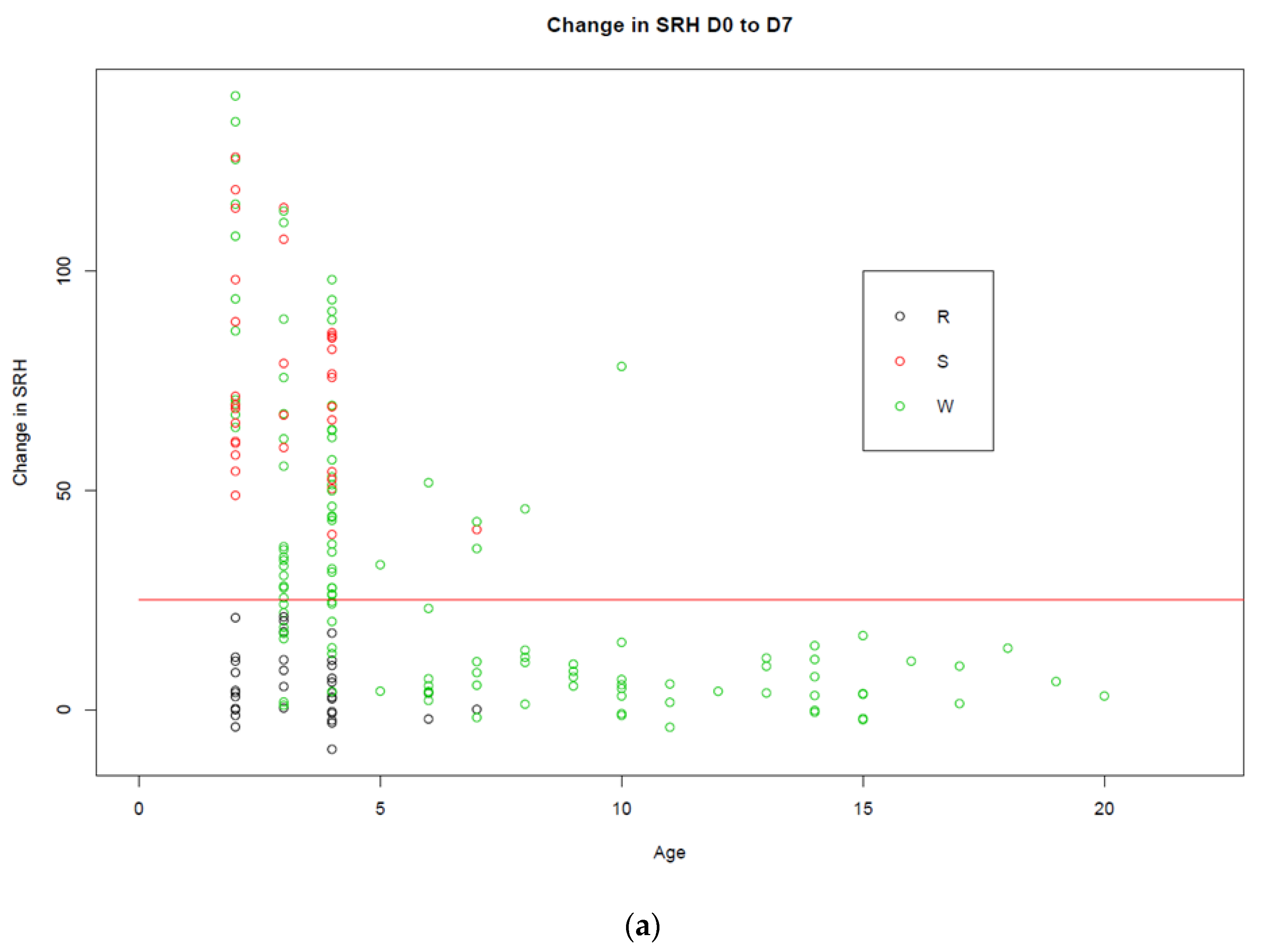
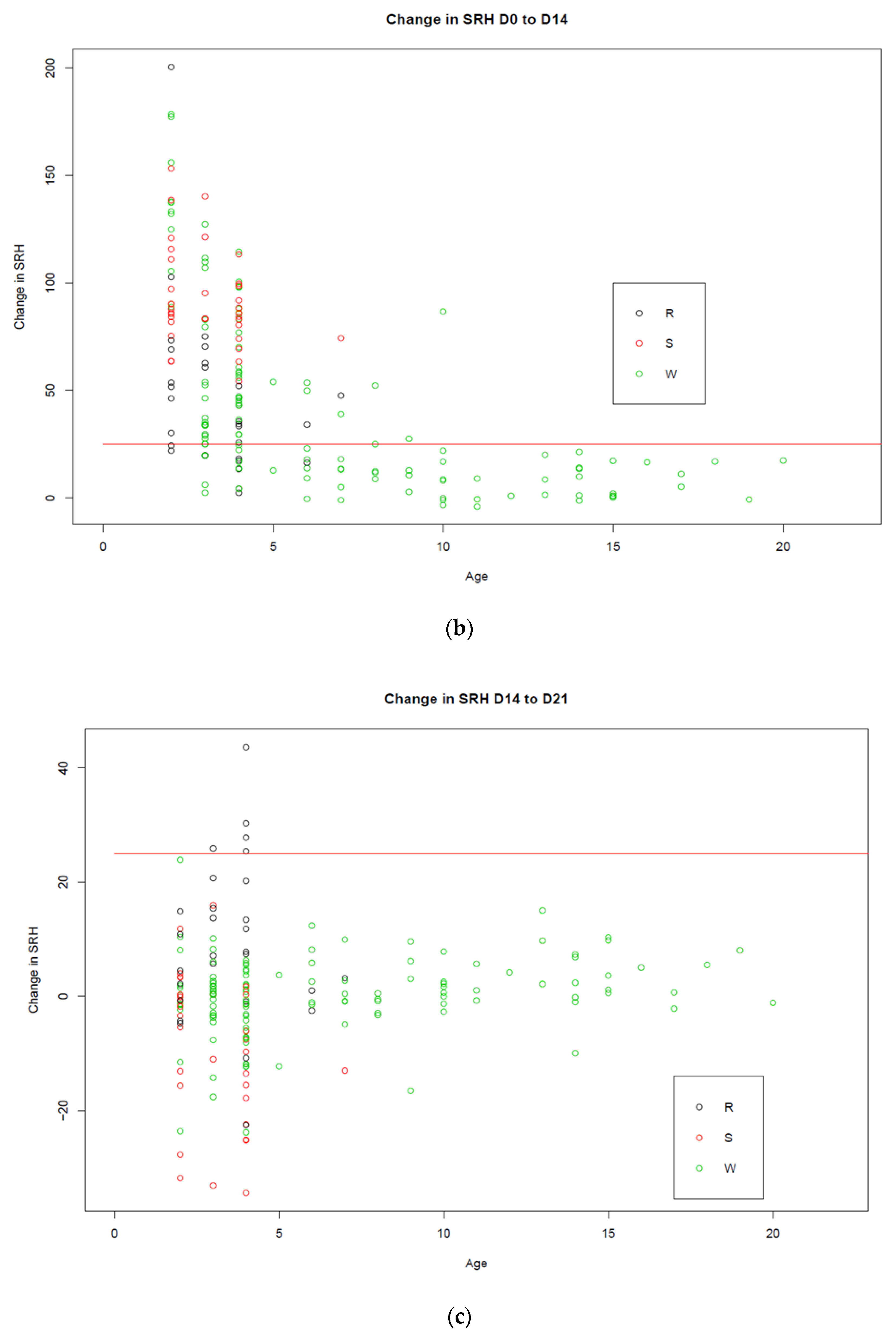
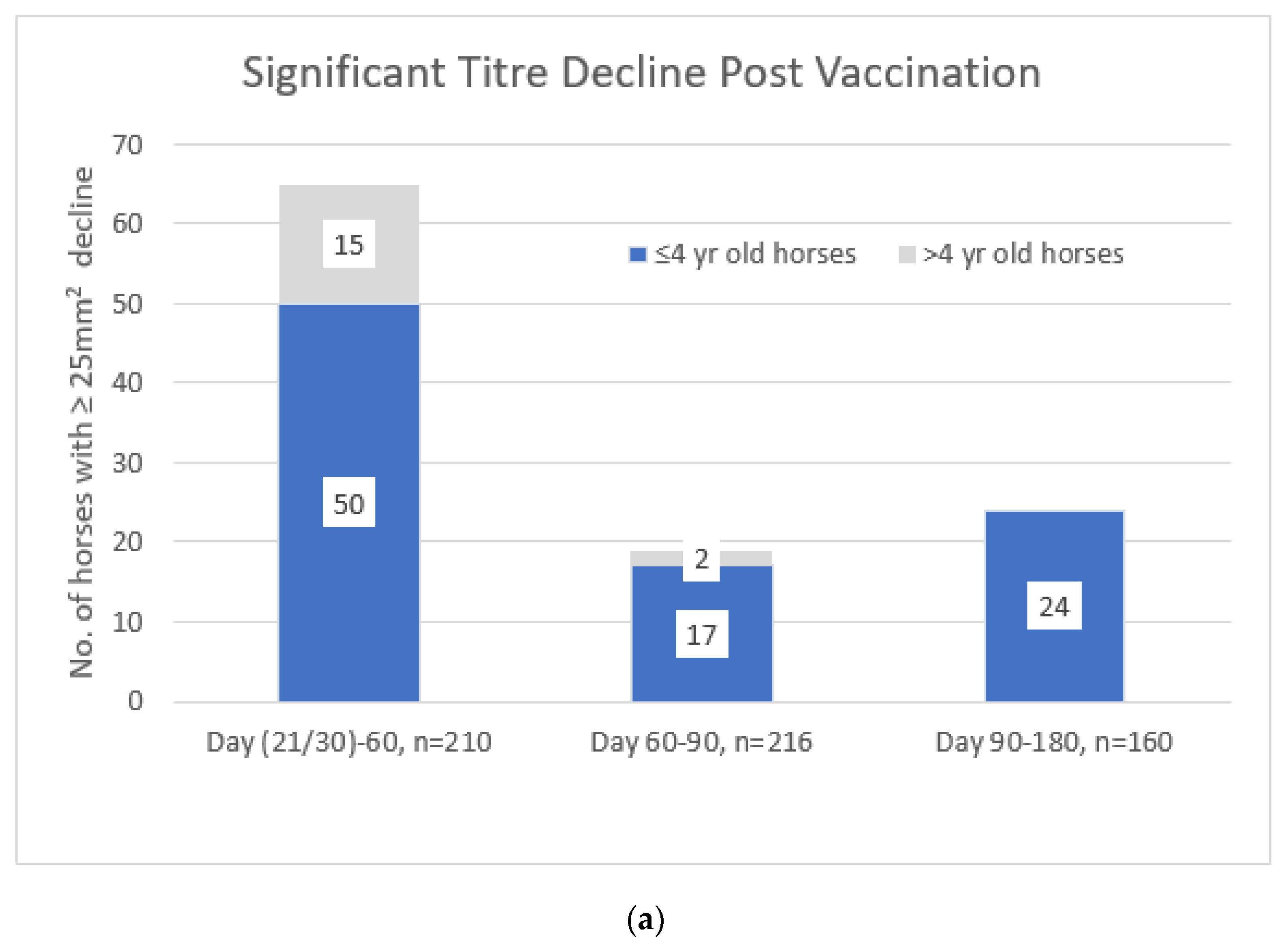
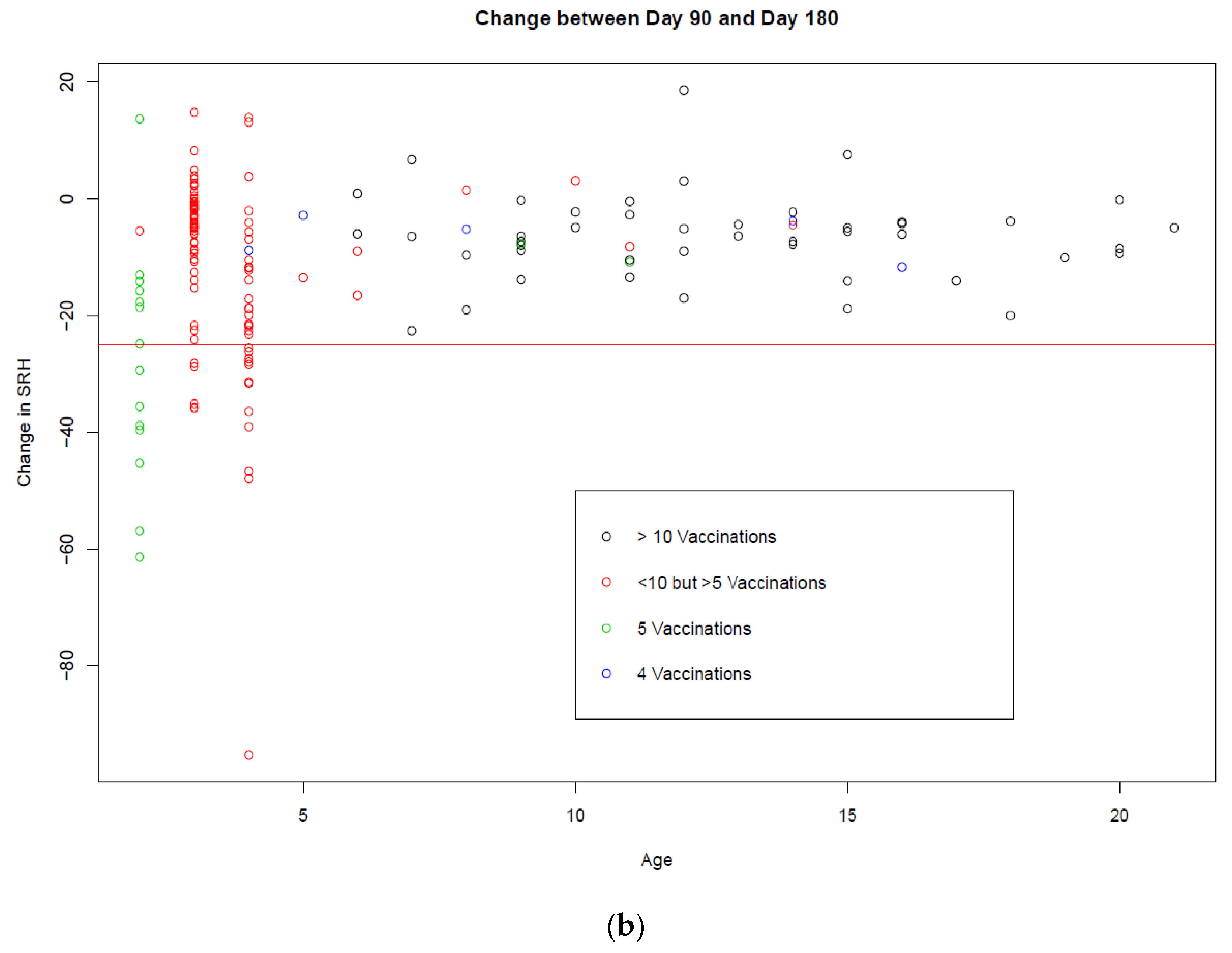
3.1. Trial A
3.2. Trial B
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cullinane, A.; Newton, J.R. Equine influenza—A global perspective. Vet. Microbiol. 2013, 167, 205–214. [Google Scholar] [CrossRef]
- Cowled, B.; Ward, M.P.; Hamilton, S.; Garner, G. The equine influenza epidemic in Australia: Spatial and temporal descriptive analyses of a large propagating epidemic. Prev. Vet. Med. 2009, 92, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Firestone, S.M.; Schemann, K.A.; Toribio, J.A.; Ward, M.P.; Dhand, N.K. A case-control study of risk factors for equine influenza spread onto horse premises during the 2007 epidemic in Australia. Prev. Vet. Med. 2011, 100, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.B.; Dagley, K.; Tainsh, J. Insights into the economic consequences of the 2007 equine influenza outbreak in Australia. Aust. Vet. J. 2011, 89, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.G.; Watkins, K.L.; Li, P.H.; Shortridge, K.F. Outbreak of equine influenza among horses in Hong Kong during 1992. Vet. Rec. 1995, 136, 531–536. [Google Scholar] [CrossRef]
- Yamanaka, T.; Niwa, H.; Tsujimura, K.; Kondo, T.; Matsumura, T. Epidemic of equine influenza among vaccinated racehorses in Japan in 2007. J. Vet. Med. Sci. 2008, 70, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, A.J.; Stevens, K.B.; Bosman, P.P. The circumstances surrounding the outbreak and spread of equine influenza in South Africa. Rev. Sci. Tech. 1999, 18, 179–185. [Google Scholar] [CrossRef]
- King, E.; Macdonald, D. Report of the Board of Inquiry appointed by the Board of the National Horseracing Authority to conduct enquiry into the causes of the equine influenza which started in the Western cape in early December 2003 and spread to the Eastern Cape and Gauteng. Aust. Equine Vet. 2004, 23, 139–142. [Google Scholar]
- Gahan, J.; Garvey, M.; Gildea, S.; Gur, E.; Kagankaya, A.; Cullinane, A. Whole-genome sequencing and antigenic analysis of the first equine influenza virus identified in Turkey. Influenza Other Respir. Viruses 2018, 12, 374–382. [Google Scholar] [CrossRef]
- Gahan, J.; Garvey, M.; Asmah Abd Samad, R.; Cullinane, A. Whole Genome Sequencing of the First H3N8 Equine Influenza Virus Identified in Malaysia. Pathogens 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Olguin-Perglione, C.; Vissani, M.A.; Alamos, F.; Tordoya, M.S.; Barrandeguy, M. Multifocal outbreak of equine influenza in vaccinated horses in Argentina in 2018: Epidemiological aspects and molecular characterisation of the involved virus strains. Equine Vet. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE). OIE Expert Surveillance Panel on Equine Influenza Vaccine Composition, OIE Headquarters, 4 April 2019. Available online: https://oiebulletin.com/?officiel=08-4-1-2019-2-panel-en (accessed on 16 December 2019).
- Fougerolle, S.; Fortier, C.; Legrand, L.; Jourdan, M.; Marcillaud-Pitel, C.; Pronost, S.; Paillot, R. Success and Limitation of Equine Influenza Vaccination: The First Incursion in a Decade of a Florida Clade 1 Equine Influenza Virus that Shakes Protection Despite High Vaccine Coverage. Vaccines 2019, 7, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, M.; Munstermann, S.; de Guindos, I.; Timoney, P. Equine disease events resulting from international horse movements: Systematic review and lessons learned. Equine Vet. J. 2016, 48, 641–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perglione, C.O.; Gildea, S.; Rimondi, A.; Mino, S.; Vissani, A.; Carossino, M.; Cullinane, A.; Barrandeguy, M. Epidemiological and virological findings during multiple outbreaks of equine influenza in South America in 2012. Influenza Other Respir. Viruses 2016, 10, 37–46. [Google Scholar] [CrossRef]
- Watson, J.; Daniels, P.; Kirkland, P.; Carroll, A.; Jeggo, M. The 2007 outbreak of equine influenza in Australia: Lessons learned for international trade in horses. Rev. Sci. Tech. 2011, 30, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Woodward, A.L.; Rash, A.S.; Blinman, D.; Bowman, S.; Chambers, T.M.; Daly, J.M.; Damiani, A.; Joseph, S.; Lewis, N.; McCauley, J.W.; et al. Development of a surveillance scheme for equine influenza in the UK and characterisation of viruses isolated in Europe, Dubai and the USA from 2010–2012. Vet. Microbiol. 2014, 169, 113–127. [Google Scholar] [CrossRef] [Green Version]
- World Organisation for Animal Health (OIE). OIE Terrestrial Animal Health Code, 2019. Paris, France. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_eiv.pdf (accessed on 16 December 2019).
- Daly, J.; Sindle, T.; Tearle, J.; Barquero, N.; Newton, J.; Corning, S. Equine influenza vaccine containing older H3N8 strains offers protection against A/eq/South Africa/4/03 (H3N8) strain in a short-term vaccine efficacy study. Equine Vet. J. 2007, 39, 446–450. [Google Scholar] [CrossRef]
- Bryant, N.A.; Paillot, R.; Rash, A.S.; Medcalf, E.; Montesso, F.; Ross, J.; Watson, J.; Jeggo, M.; Lewis, N.S.; Newton, J.R. Comparison of two modern vaccines and previous influenza infection against challenge with an equine influenza virus from the Australian 2007 outbreak. Vet. Res. 2010, 41. [Google Scholar] [CrossRef] [Green Version]
- Paillot, R. A Systematic Review of Recent Advances in Equine Influenza Vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef]
- Gildea, S.; Arkins, S.; Cullinane, A. A comparative antibody study of the potential susceptibility of Thoroughbred and non-Thoroughbred horse populations in Ireland to equine influenza virus. Influenza Other Respir. Viruses 2010, 4, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Newton, J.R.; Townsend, H.G.; Wood, J.L.; Sinclair, R.; Hannant, D.; Mumford, J.A. Immunity to equine influenza: Relationship of vaccine-induced antibody in young Thoroughbred racehorses to protection against field infection with influenza A/equine-2 viruses (H3N8). Equine Vet. J. 2000, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 20 February 2020).
- Mumford, J.; Wood, J. WHO/OIE meeting: Consultation on newly emerging strains of equine influenza. 18–19 May 1992, Animal Health Trust, Newmarket, Suffolk, UK. Vaccine 1993, 11, 1172–1175. [Google Scholar] [CrossRef]
- Luna, L.K.; Panning, M.; Grywna, K.; Pfefferle, S.; Drosten, C. Spectrum of viruses and atypical bacteria in intercontinental air travelers with symptoms of acute respiratory infection. J. Infect. Dis. 2007, 195, 675–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leadon, D.; Waran, N.; Herholz, C.; Klay, M. Veterinary management of horse transport. Vet. Ital. 2008, 44, 149–163. [Google Scholar] [PubMed]
- Cullinane, A. Equine influenza and air transport. Equine Vet. Educ. 2014, 26, 456–457. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). World Health Organisation: Ten Threats to Global Health in 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 16 December 2019).
- Andersen, S.A.; Petersen, H.H.; Ersbøll, A.K.; Falk-Rønne, J.; Jacobsen, S. Vaccination elicits a prominent acute phase response in horses. Vet. J. 2012, 191, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, L.J.; Netherwood, K.A.; Norris, M.S.; Behrens, N.E.; Shao, M.X. Equine IgE responses to non-viral vaccine components. Vaccine 2012, 30, 7615–7620. [Google Scholar] [CrossRef]
- Paillot, R.; Kydd, J.H.; Sindle, T.; Hannant, D.; Edlund Toulemonde, C.; Audonnet, J.C.; Minke, J.M.; Daly, J.M. Antibody and IFN-gamma responses induced by a recombinant canarypox vaccine and challenge infection with equine influenza virus. Vet. Immunol. Immunopathol. 2006, 112, 225–233. [Google Scholar] [CrossRef]
- Paillot, R.; Grimmett, H.; Elton, D.; Daly, J.M. Protection, systemic IFNgamma, and antibody responses induced by an ISCOM-based vaccine against a recent equine influenza virus in its natural host. Vet. Res. 2008, 39, 21. [Google Scholar] [CrossRef] [Green Version]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.M.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, IFNgamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef]
- Gildea, S.; Quinlivan, M.; Murphy, B.A.; Cullinane, A. Humoral response and antiviral cytokine expression following vaccination of thoroughbred weanlings—A blinded comparison of commercially available vaccines. Vaccine 2013, 31, 5216–5222. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.A. Collaborative study for the establishment of three European Pharmacopoeia Biological Reference Preparations for equine influenza horse antiserum. Pharmeuropa 2000, 1, 7–21. [Google Scholar]
- Daly, J.; Daas, A.; Behr-Gross, M. Collaborative study for the establishment of a candidate equine influenza subtype 2 American-like strain A/EQ/South Africa/4/03-horse antiserum biological reference preparation. Pharmeuropa Bio 2007, 2007, 7–14. [Google Scholar] [PubMed]
- Mumford, J.A.; Wood, J. Establishing an acceptability threshold for equine influenza vaccines. Dev. Biol. Stand. 1992, 79, 137–146. [Google Scholar]
- Mumford, J.A.; Jessett, D.; Dunleavy, U.; Wood, J.; Hannant, D.; Sundquist, B.; Cook, R.F. Antigenicity and immunogenicity of experimental equine influenza ISCOM vaccines. Vaccine 1994, 12, 857–863. [Google Scholar] [CrossRef]
- Mumford, J.A.; Wilson, H.; Hannant, D.; Jessett, D.M. Antigenicity and immunogenicity of equine influenza vaccines containing a Carbomer adjuvant. Epidemiol. Infect. 1994, 112, 421–437. [Google Scholar] [CrossRef] [Green Version]
- Newton, J.R.; Verheyen, K.; Wood, J.L.; Yates, P.J.; Mumford, J.A. Equine influenza in the United Kingdom in 1998. Vet. Rec. 1999, 145, 449–452. [Google Scholar] [CrossRef]
- Haaheim, L.R.; Schild, G.C. Antibodies to the strain-specific and cross-reactive determinants of the haemagglutinin of influenza H3N2 viruses. Antiviral activities of the antibodies in biological systems. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. 1980, 88, 335–340. [Google Scholar]
- Yates, P.; Mumford, J.A. Equine influenza vaccine efficacy: The significance of antigenic variation. Vet. Microbiol. 2000, 74, 173–177. [Google Scholar] [CrossRef]
- Livesay, G.J.; O’Neill, T.; Hannant, D.; Yadav, M.P.; Mumford, J.A. The outbreak of equine influenza (H3N8) in the United Kingdom in 1989: Diagnostic use of an antigen capture ELISA. Vet. Rec. 1993, 133, 515–519. [Google Scholar] [CrossRef]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following annual booster vaccination of National Hunt horses—A randomised blind study. Vaccine 2011, 29, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; El-Hage, C.M. The Use of a Recombinant Canarypox-Based Equine Influenza Vaccine during the 2007 Australian Outbreak: A Systematic Review and Summary. Pathogens 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvin, P.; Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. The evaluation of a nucleoprotein ELISA for the detection of equine influenza antibodies and the differentiation of infected from vaccinated horses (DIVA). Influenza Other Respir. Viruses 2013, 7, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following primary vaccination of Thoroughbred weanlings—A randomised blind study. Vaccine 2011, 29, 9214–9223. [Google Scholar] [CrossRef] [PubMed]





| Recommendation | FEI 1 | IFHA 2 | OIE 3 |
|---|---|---|---|
| Boost before export/competition | For horses competing, the last booster must be given within 6 months + 21 days (and not within 7 days) before arrival at the event | During the 60 days immediately prior to export from its country of origin, but not within 14 days of export. | Immunised between 21 and 90 days before shipment |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cullinane, A.; Gahan, J.; Walsh, C.; Nemoto, M.; Entenfellner, J.; Olguin-Perglione, C.; Garvey, M.; Huang Fu, T.Q.; Venner, M.; Yamanaka, T.; et al. Evaluation of Current Equine Influenza Vaccination Protocols Prior to Shipment, Guided by OIE Standards. Vaccines 2020, 8, 107. https://doi.org/10.3390/vaccines8010107
Cullinane A, Gahan J, Walsh C, Nemoto M, Entenfellner J, Olguin-Perglione C, Garvey M, Huang Fu TQ, Venner M, Yamanaka T, et al. Evaluation of Current Equine Influenza Vaccination Protocols Prior to Shipment, Guided by OIE Standards. Vaccines. 2020; 8(1):107. https://doi.org/10.3390/vaccines8010107
Chicago/Turabian StyleCullinane, Ann, Jacinta Gahan, Cathal Walsh, Manabu Nemoto, Johanna Entenfellner, Cecilia Olguin-Perglione, Marie Garvey, Tao Qi Huang Fu, Monica Venner, Takashi Yamanaka, and et al. 2020. "Evaluation of Current Equine Influenza Vaccination Protocols Prior to Shipment, Guided by OIE Standards" Vaccines 8, no. 1: 107. https://doi.org/10.3390/vaccines8010107





