Genetic Variation in the Magnitude and Longevity of the IgG Subclass Response to a Diphtheria-Tetanus-Acellular Pertussis (DTaP) Vaccine in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Mice and Vaccination
2.2. Serology by ELISA
2.3. Genetic Association Mapping
2.4. Statistical Analysis
3. Results
3.1. Significant Inter-Strain Variability for IgG1, IgG2b, and IgG3
3.2. Contrasting Patterns of Antibody Titers and Longevity Correlations Between IgG1, IgG2b, and IgG3
3.3. Different Patterns of Antigen-Specific Responses Among IgG1, IgG2b, and IgG3 for Magnitude and Longevity
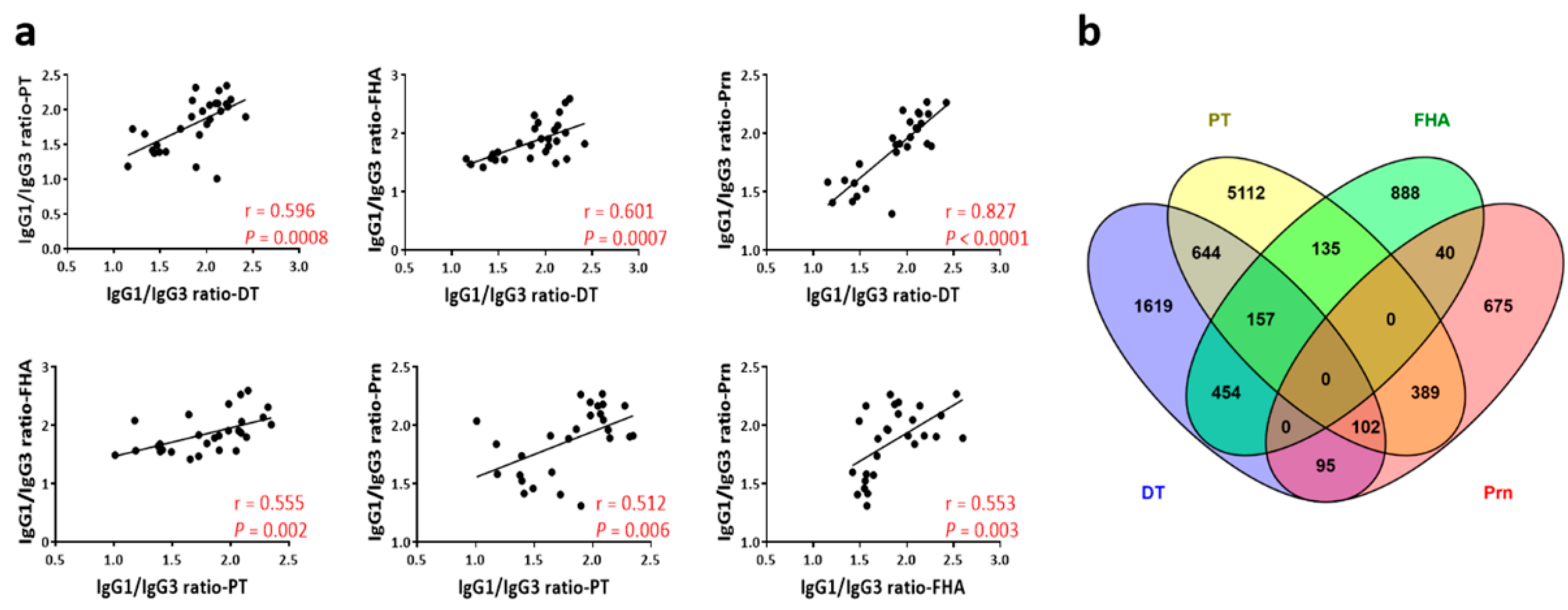
3.4. Utilization of the Ratio of IgG1 and IgG3 Titers to Identify Th1 and Th2-Prone Mouse strains
3.5. The Effect of TLR4 Signaling on IgG Subclasses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Jouvin-Marche, E.; Morgado, M.G.; Leguern, C.; Voegtle, D.; Bonhomme, F.; Cazenave, P.A. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics 1989, 29, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Morgado, M.G.; Cam, P.; Gris-Liebe, C.; Cazenave, P.A.; Jouvin-Marche, E. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 1989, 8, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Goldschmidt, T.; Salter, H. Possible allelic structure of IgG2a and IgG2c in mice. Mol. Immunol. 2012, 50, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Silva, A.; Lew, A.M. The Igh-1 sequence of the non-obese diabetic (NOD) mouse assigns it to the IgG2c isotype. Immunogenetics 1997, 46, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Ravetch, J.V. Fcgamma receptor function and the design of vaccination strategies. Immunity 2017, 47, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Leatherbarrow, R.J.; Dwek, R.A. Binding of complement subcomponent C1q to mouse IgG1, IgG2a and IgG2b: A novel C1q binding assay. Mol. Immunol. 1984, 21, 321–327. [Google Scholar] [CrossRef]
- Bruhns, P.; Jonsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, I.; Yoshikawa, K.; Kinoshita, K.; Muramatsu, M.; Nagaoka, H.; Honjo, T. Activation-induced cytidine deaminase links class switch recombination and somatic hypermutation. Ann. N. Y. Acad. Sci. 2003, 987, 1–8. [Google Scholar] [CrossRef]
- Snapper, C.M.; Mond, J.J. Towards a comprehensive view of immunoglobulin class switching. Immunol. Today 1993, 14, 15–17. [Google Scholar] [CrossRef]
- Snapper, C.M.; McIntyre, T.M.; Mandler, R.; Pecanha, L.M.; Finkelman, F.D.; Lees, A.; Mond, J.J. Induction of IgG3 secretion by interferon gamma: A model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 1992, 175, 1367–1371. [Google Scholar] [CrossRef]
- Snapper, C.M.; Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef]
- Stavnezer, J. Regulation of antibody production and class switching by TGF-beta. J. Immunol. 1995, 155, 1647–1651. [Google Scholar]
- Germann, T.; Bongartz, M.; Dlugonska, H.; Hess, H.; Schmitt, E.; Kolbe, L.; Kolsch, E.; Podlaski, F.J.; Gately, M.K.; Rude, E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 1995, 25, 823–829. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Van Rie, A.; Salmaso, S.; Englund, J.A. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 2005, 24, S58–S61. [Google Scholar] [CrossRef]
- Dalby, T.; Petersen, J.W.; Harboe, Z.B.; Krogfelt, K.A. Antibody responses to pertussis toxin display different kinetics after clinical Bordetella pertussis infection than after vaccination with an acellular pertussis vaccine. J. Med. Microbiol. 2010, 59, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Barkoff, A.M.; Grondahl-Yli-Hannuksela, K.; Vuononvirta, J.; Mertsola, J.; Kallonen, T.; He, Q. Differences in avidity of IgG antibodies to pertussis toxin after acellular pertussis booster vaccination and natural infection. Vaccine 2012, 30, 6897–6902. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; de Blasio, B.F.; Storsaeter, J. Short-lived immunity against pertussis, age-specific routes of transmission, and the utility of a teenage booster vaccine. Vaccine 2012, 30, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.L.; Jacobson, R.M.; Poland, G.A.; Jacobsen, S.J.; Pankratz, V.S. Twin studies of immunogenicity--determining the genetic contribution to vaccine failure. Vaccine 2001, 19, 2434–2439. [Google Scholar] [CrossRef]
- Newport, M.J.; Goetghebuer, T.; Weiss, H.A.; Whittle, H.; Siegrist, C.A.; Marchant, A. Genetic regulation of immune responses to vaccines in early life. Genes Immun. 2004, 5, 122–129. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.; Pollard, A.J. Characterizing vaccine responses using host genomic and transcriptomic analysis. Clin. Infect. Dis. 2013, 57, 860–869. [Google Scholar] [CrossRef]
- Hohler, T.; Reuss, E.; Evers, N.; Dietrich, E.; Rittner, C.; Freitag, C.M.; Vollmar, J.; Schneider, P.M.; Fimmers, R. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: A vaccination study in twins. Lancet 2002, 360, 991–995. [Google Scholar] [CrossRef]
- Davis, R.C.; van Nas, A.; Bennett, B.; Orozco, L.; Pan, C.; Rau, C.D.; Eskin, E.; Lusis, A.J. Genome-wide association mapping of blood cell traits in mice. Mamm. Genome 2013, 24, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Jonczyk, M.S.; Simon, M.; Kumar, S.; Fernandes, V.E.; Sylvius, N.; Mallon, A.M.; Denny, P.; Andrew, P.W. Genetic factors regulating lung vasculature and immune cell functions associate with resistance to pneumococcal infection. PLoS ONE 2014, 9, e89831. [Google Scholar] [CrossRef]
- Mosley, Y.C.; Radder, J.E.; Berndt, A.; HogenEsch, H. Genome-wide association mapping of the antibody response to Diphtheria, Tetanus and acellular Pertussis vaccine in mice. J. Infect. Dis. 2017, 215, 466–474. [Google Scholar] [CrossRef]
- Payseur, B.A.; Place, M. Prospects for association mapping in classical inbred mouse strains. Genetics 2007, 175, 1999–2008. [Google Scholar] [CrossRef]
- Manenti, G.; Galvan, A.; Pettinicchio, A.; Trincucci, G.; Spada, E.; Zolin, A.; Milani, S.; Gonzalez-Neira, A.; Dragani, T.A. Mouse genome-wide association mapping needs linkage analysis to avoid false-positive Loci. PLoS Genet. 2009, 5, e1000331. [Google Scholar] [CrossRef]
- Flint, J.; Eskin, E. Genome-wide association studies in mice. Nat. Rev. Genet. 2012, 13, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Storsaeter, J.; Hallander, H.O.; Gustafsson, L.; Olin, P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998, 16, 1907–1916. [Google Scholar] [CrossRef]
- Cherry, J.D.; Gornbein, J.; Heininger, U.; Stehr, K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 1998, 16, 1901–1906. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Hellwig, S.M.; Hozbor, D.F.; Leusen, J.; van der Pol, W.L.; van de Winkel, J.G. Fc receptor-mediated immunity against Bordetella pertussis. J. Immunol. 2001, 167, 6545–6551. [Google Scholar] [CrossRef]
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013, 9, e1003264. [Google Scholar] [CrossRef]
- Raeven, R.H.; van der Maas, L.; Tilstra, W.; Uittenbogaard, J.P.; Bindels, T.H.; Kuipers, B.; van der Ark, A.; Pennings, J.L.; van Riet, E.; Jiskoot, W.; et al. Immunoproteomic profiling of Bordetella pertussis Outer Membrane Vesicle vaccine reveals broad and balanced humoral immunogenicity. J. Proteome Res. 2015, 14, 2929–2942. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Macatonia, S.E.; O’Garra, A.; Murphy, K.M. T cell genetic background determines default T helper phenotype development in vitro. J. Exp. Med. 1995, 181, 713–721. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Tsang, J.S. Utilizing population variation, vaccination, and systems biology to study human immunology. Trends Immunol. 2015, 36, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Warfel, J.M.; Edwards, K.M. Pertussis vaccines and the challenge of inducing durable immunity. Curr. Opin. Immunol. 2015, 35, 48–54. [Google Scholar] [CrossRef]
- Allen, A.C.; Mills, K.H. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev. Vaccines 2014, 13, 1253–1264. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Casella, C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017, 47, 17–25. [Google Scholar] [CrossRef]
- Authier, F.J.; Sauvat, S.; Christov, C.; Chariot, P.; Raisbeck, G.; Poron, M.F.; Yiou, F.; Gherardi, R. AlOH3-adjuvanted vaccine-induced macrophagic myofasciitis in rats is influenced by the genetic background. Neuromuscul. Disord. NMD 2006, 16, 347–352. [Google Scholar] [CrossRef]
- Brewer, J.M.; Conacher, M.; Satoskar, A.; Bluethmann, H.; Alexander, J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur. J. Immunol. 1996, 26, 2062–2066. [Google Scholar] [CrossRef]
- Comoy, E.E.; Capron, A.; Thyphronitis, G. In vivo induction of type 1 and 2 immune responses against protein antigens. Int. Immunol. 1997, 9, 523–531. [Google Scholar] [CrossRef]
- Martin, R.M.; Brady, J.L.; Lew, A.M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 1998, 212, 187–192. [Google Scholar] [CrossRef]
- Baumgarth, N.; Kelso, A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J. Virol. 1996, 70, 4411–4418. [Google Scholar] [Green Version]
- Huang, S.; Hendriks, W.; Althage, A.; Hemmi, S.; Bluethmann, H.; Kamijo, R.; Vilcek, J.; Zinkernagel, R.M.; Aguet, M. Immune response in mice that lack the interferon-gamma receptor. Science 1993, 259, 1742–1745. [Google Scholar] [CrossRef]
- Hendrikx, L.H.; Schure, R.M.; Ozturk, K.; de Rond, L.G.; de Greeff, S.C.; Sanders, E.A.; Berbers, G.A.; Buisman, A.M. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine 2011, 29, 6874–6880. [Google Scholar] [CrossRef]
- van der Lee, S.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole-cell or acellular pertussis vaccination in infancy determines IgG subclass profiles to DTaP booster vaccination. Vaccine 2018, 36, 220–226. [Google Scholar] [CrossRef]
- Giammanco, A.; Taormina, S.; Chiarini, A.; Dardanoni, G.; Stefanelli, P.; Salmaso, S.; Mastrantonio, P. Analogous IgG subclass response to pertussis toxin in vaccinated children, healthy or affected by whooping cough. Vaccine 2003, 21, 1924–1931. [Google Scholar] [CrossRef]
- Lundgren, M.; Persson, U.; Larsson, P.; Magnusson, C.; Smith, C.I.; Hammarstrom, L.; Severinson, E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur. J. Immunol. 1989, 19, 1311–1315. [Google Scholar] [CrossRef]
- Ishizaka, A.; Sakiyama, Y.; Nakanishi, M.; Tomizawa, K.; Oshika, E.; Kojima, K.; Taguchi, Y.; Kandil, E.; Matsumoto, S. The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin. Exp. Immunol. 1990, 79, 392–396. [Google Scholar] [CrossRef]
- Fahey, J.L.; Robinson, A.G. Factors controlling serum gamma-globulin concentration. J. Exp. Med. 1963, 118, 845–868. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Strober, W. Metabolism of immunoglobulins. Prog. Allergy 1969, 13, 1–110. [Google Scholar]
- Brambell, F.W.; Hemmings, W.A.; Morris, I.G. A Theoretical Model of Gamma-Globulin Catabolism. Nature 1964, 203, 1352–1354. [Google Scholar] [CrossRef]
- Junghans, R.P.; Anderson, C.L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 5512–5516. [Google Scholar] [CrossRef]
- Israeli, E.; Agmon-Levin, N.; Blank, M.; Shoenfeld, Y. Macrophagic myofaciitis a vaccine (alum) autoimmune-related disease. Clin. Rev. Allergy Immunol. 2011, 41, 163–168. [Google Scholar] [CrossRef]
- Ghetie, V.; Hubbard, J.G.; Kim, J.K.; Tsen, M.F.; Lee, Y.; Ward, E.S. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 1996, 26, 690–696. [Google Scholar] [CrossRef]
- Challa, D.K.; Velmurugan, R.; Ober, R.J.; Sally Ward, E. FcRn: From molecular interactions to regulation of IgG pharmacokinetics and functions. Curr. Top. Microbiol. Immunol. 2014, 382, 249–272. [Google Scholar] [CrossRef]
- Ghetie, V.; Ward, E.S. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu. Rev. Immunol. 2000, 18, 739–766. [Google Scholar] [CrossRef]
- Ober, R.J.; Radu, C.G.; Ghetie, V.; Ward, E.S. Differences in promiscuity for antibody-FcRn interactions across species: Implications for therapeutic antibodies. Int. Immunol. 2001, 13, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.L.; Hoebe, K.; Duong, B.; Ota, T.; Martin, C.; Beutler, B.; Nemazee, D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 2006, 314, 1936–1938. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, S.M.; Long, E.M.; Hickey, M.J.; Andonegui, G.; Lapointe, B.M.; Zanardo, R.C.; Bonder, C.; James, W.G.; Robbins, S.M.; Kubes, P. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J. Immunol. 2004, 173, 7070–7077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yang, D.; Chen, Q.; Leifer, C.A.; Segal, D.M.; Su, S.B.; Caspi, R.R.; Howard, Z.O.; Oppenheim, J.J. Induction of dendritic cell maturation by pertussis toxin and its B subunit differentially initiate Toll-like receptor 4-dependent signal transduction pathways. Exp. Hematol. 2006, 34, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Nasso, M.; Fedele, G.; Spensieri, F.; Palazzo, R.; Costantino, P.; Rappuoli, R.; Ausiello, C.M. Genetically detoxified pertussis toxin induces Th1/Th17 immune response through MAPKs and IL-10-dependent mechanisms. J. Immunol. 2009, 183, 1892–1899. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosley, Y.-Y.C.; Radder, J.E.; HogenEsch, H. Genetic Variation in the Magnitude and Longevity of the IgG Subclass Response to a Diphtheria-Tetanus-Acellular Pertussis (DTaP) Vaccine in Mice. Vaccines 2019, 7, 124. https://doi.org/10.3390/vaccines7040124
Mosley Y-YC, Radder JE, HogenEsch H. Genetic Variation in the Magnitude and Longevity of the IgG Subclass Response to a Diphtheria-Tetanus-Acellular Pertussis (DTaP) Vaccine in Mice. Vaccines. 2019; 7(4):124. https://doi.org/10.3390/vaccines7040124
Chicago/Turabian StyleMosley, Yung-Yi C., Josiah E. Radder, and Harm HogenEsch. 2019. "Genetic Variation in the Magnitude and Longevity of the IgG Subclass Response to a Diphtheria-Tetanus-Acellular Pertussis (DTaP) Vaccine in Mice" Vaccines 7, no. 4: 124. https://doi.org/10.3390/vaccines7040124





