Immune History and Influenza Vaccine Effectiveness
Abstract
:1. Introduction
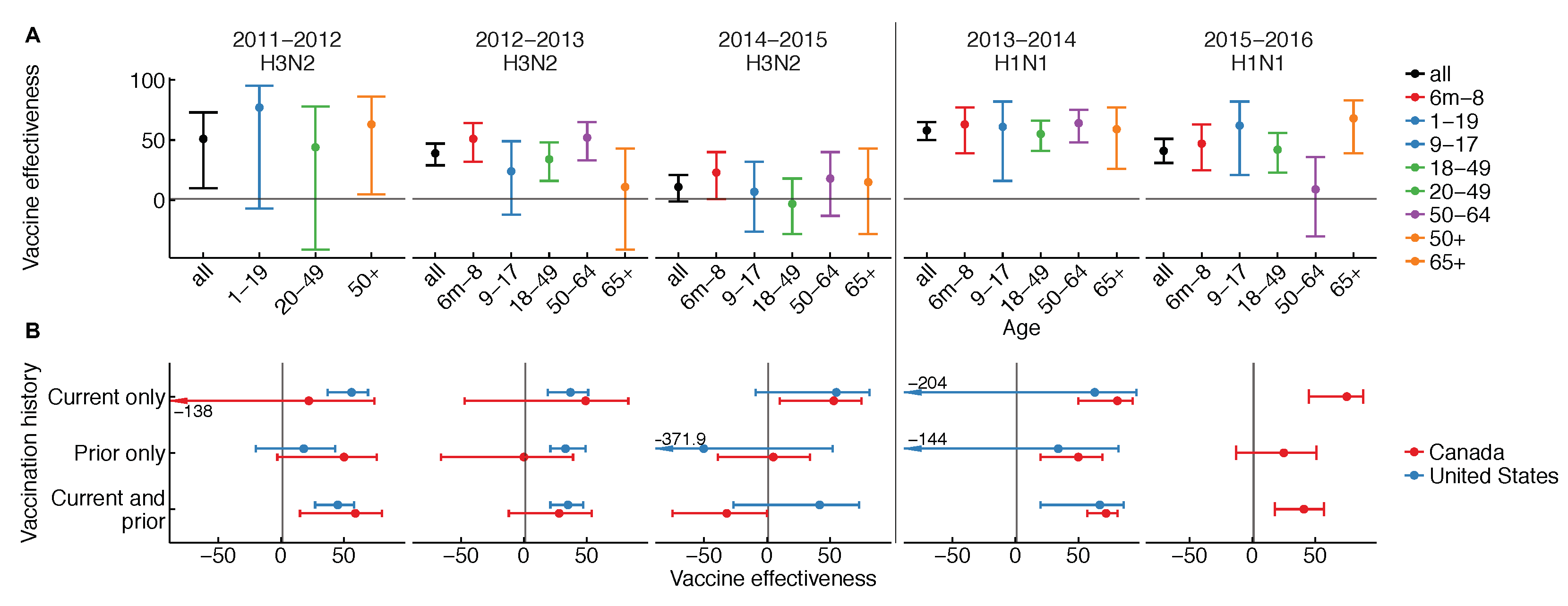
2. What Do We Expect, and What Do We See?
3. Are Our Measures of Vaccine Effectiveness Part of the Problem?
4. Biological Explanations: Original Antigenic Sin
5. Other Biological Explanations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OAS | Original antigenic sin |
| OR | Odds ratio |
| TND | Test negative design |
| VE | Vaccine efficacy or effectiveness (defined here as the vaccine direct effect on susceptibility to infection and/or disease) |
Appendix A
| Season | VE Type | Notes | Source |
|---|---|---|---|
| 2011–2012 | Age | All ages | Canada [91] |
| Vaccination history | ≥9 years, all flu | U.S. [92] | |
| ≥2 years | Canada [91] | ||
| 2012–2013 | Age | All ages | U.S. [93] |
| Vaccination history | ≥9 years | U.S. [93] | |
| ≥9 years | Canada [67] | ||
| 2013-2014 | Age | ≥6 months | U.S. [94] |
| Vaccination history | ≥9 years | U.S. [95] | |
| ≥2 years | Canada [96] | ||
| 2014–2015 | Age | ≥6 months | U.S. [97] |
| Vaccination history | ≥18 years | U.S. [98] | |
| ≥2 years | Canada [17] | ||
| 2015–2016 | Age | ≥6 months | U.S. [97] |
| Vaccination history | ≥9 years | Canada [12] |
Appendix B. Measuring VE against Influenza
References
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Erbelding, E.J.; Post, D.; Stemmy, E.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Kieke, B.A.; Donahue, J.G.; Greenlee, R.T.; Balish, A.; Foust, A.; Lindstrom, S.; Shay, D.K. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J. Infect. Dis. 2009, 199, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Cate, T.R.; Couch, R.B.; Huggins, L.L.; Hess, K.R. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997, 15, 1114–1122. [Google Scholar] [CrossRef]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Perez, S.D.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.D.; Barjesteh, N.; Mapletoft, J.P.; Miller, M.S. “Gnothi Seauton”: Leveraging the Host Response to Improve Influenza Virus Vaccine Efficacy. Vaccines 2018, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Ramsay, L.C.; Buchan, S.A.; Stirling, R.G.; Cowling, B.J.; Feng, S.; Kwong, J.C.; Warshawsky, B.F. The impact of repeated vaccination on influenza vaccine effectiveness: A systematic review and meta-analysis. BMC Med. 2017, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Skowronski, D.M.; McLean, H.Q.; Chambers, C.; Sundaram, M.E.; De Serres, G. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Rev. Vaccines 2017, 16, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Cowling, B.J.; Thompson, M.G.; Shay, D.K.; Monto, A.S. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 2013, 56, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Smith, C.; Garten, R.J.; Levine, M.Z.; Chung, J.R.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Influence of Birth Cohort on Effectiveness of 2015–2016 Influenza Vaccine Against Medically Attended Illness Due to 2009 Pandemic Influenza A (H1N1) Virus in the United States. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; Charest, H.; et al. Beyond Antigenic Match: Possible Agent-Host and Immuno-epidemiological Influences on Influenza Vaccine Effectiveness During the 2015–2016 Season in Canada. J. Infect. Dis. 2017, 216, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Chung, J.R.; Thaker, S.N.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; et al. Interim Estimates of 2016–2017 Seasonal Influenza Vaccine Effectiveness- United States, February 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Chung, J.R.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. Am. J. Transplant. 2018, 18, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Meece, J.K.; McClure, D.L.; Friedrich, T.C.; Belongia, E.A. Impact of repeated vaccination on vaccine effectiveness against influenza A (H3N2) and B during 8 seasons. Clin. Infect. Dis. 2014, 59, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Komori, K.; Suzuki, M.; Kishikawa, T.; Yasaka, T.; Ariyoshi, K. Dose-Dependent Negative Effects of Prior Multiple Vaccinations against Influenza A and Influenza B among School Children: A Study of Kamigoto Island in Japan during the 2011/12, 2012/13 and 2013/14 Influenza Seasons. Clin. Infect. Dis. 2018, ciy202. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Krajden, M.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; et al. A perfect storm: Impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin. Infect. Dis. 2016, 63, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G.; Crowcroft, N.S.; Janjua, N.Z.; Boulianne, N.; Hottes, T.S.; Rosella, L.C.; Dickinson, J.A.; Gilca, R.; Sethi, P.; et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring–summer 2009: Four observational studies from Canada. PLoS Med. 2010, 7, e1000258. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Ng, S.; Ma, E.S.; Cheng, C.K.; Wai, W.; Fang, V.J.; Chan, K.H.; Ip, D.K.; Chiu, S.S.; Peiris, J.M.; et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin. Infect. Dis. 2010, 51, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Carcione, D.; Giele, C.; Goggin, L.; Kwan, K.; Smith, D.; Dowse, G.; Mak, D.B.; Effler, P. Association between 2009 seasonal influenza vaccine and influenza-like illness during the 2009 pandemic: Preliminary results of a large household transmission study in Western Australia. Eurosurveillance 2010, 15, 19616. [Google Scholar] [PubMed]
- Jefferies, S.; Earl, D.; Berry, N.; Blackmore, T.; Rooker, S.; Raymond, N.; Pritchard, A.; Weatherall, M.; Beasley, R.; Perrin, K. Effectiveness of the 2009 seasonal influenza vaccine against pandemic influenza A (H1N1) 2009 in healthcare workers in New Zealand, June–August 2009. Eurosurveillance 2011, 16, 19761. [Google Scholar] [PubMed]
- Halloran, M.E.; Haber, M.; Longini, I.M., Jr.; Struchiner, C.J. Direct and indirect effects in vaccine efficacy and effectiveness. Am. J. Epidemiol. 1991, 133, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Foppa, I.M.; Haber, M.; Ferdinands, J.M.; Shay, D.K. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013, 31, 3104–3109. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.V.; Facklam, R.R.; Fraser, D.W. Pneumococcal disease after pneumococcal vaccination: An alternative method to estimate the efficacy of pneumococcal vaccine. N. Engl. J. Med. 1980, 303, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Wacholder, S.; Silverman, D.T.; McLaughlin, J.K.; Mandel, J.S. Selection of controls in case-control studies: II. Types of controls. Am. J. Epidemiol. 1992, 135, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Nelson, J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013, 31, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Foppa, I.M.; Ferdinands, J.M.; Chaves, S.S.; Haber, M.J.; Reynolds, S.B.; Flannery, B.; Fry, A.M. The case test-negative design for studies of the effectiveness of influenza vaccine in inpatient settings. Int. J. Epidemiol. 2016, 45, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Rodrigues, L.; Fine, P. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int. J. Epidemiol. 1984, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.P.; Struchiner, C.J.; Halloran, M.E. Assessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: A case-control study. Int. J. Epidemiol. 1995, 24, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Tchetgen Tchetgen, E.J.; Cowling, B.J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am. J. Epidemiol. 2016, 184, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Jha, A.; Simonsen, L. Observational studies and the difficult quest for causality: Lessons from vaccine effectiveness and impact studies. Int. J. Epidemiol. 2016, 45, 2060–2074. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; An, Q.; Ainslie, K.E.; Haber, M.; Orenstein, W.A. A comparison of the test-negative and the traditional case-control study designs for estimation of influenza vaccine effectiveness under nonrandom vaccination. BMC Infect. Dis. 2017, 17, 757. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.E.; Shi, M.; Haber, M.; Orenstein, W.A. On the bias of estimates of influenza vaccine effectiveness from test–negative studies. Vaccine 2017, 35, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Tedijanto, C.; Cowling, B.J.; Lipsitch, M. Quantifying biases in test-negative studies of vaccine effectiveness. bioRxiv 2018, 237503. [Google Scholar] [CrossRef]
- Westreich, D.; Hudgens, M.G. Invited commentary: Beware the test-negative design. Am. J. Epidemiol. 2016, 184, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Foppa, I.M.; Fry, A.M.; Flannery, B.L.; Belongia, E.A.; Jackson, M.L. Re: “Invited Commentary: Beware the Test-Negative Design”. Am. J. Epidemiol. 2017, 185, 613. [Google Scholar] [CrossRef] [PubMed]
- Small, P.A.; Cronin, B.J. The Advisory Committee on Immunization Practices’ controversial recommendation against the use of live attenuated influenza vaccine is based on a biased study design that ignores secondary protection. Vaccine 2017, 8, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Karron, R.A.; Reingold, A.; Walter, E.B.; Bennett, N.M. The Advisory Committee on Immunization Practices recommendation regarding the use of live influenza vaccine: A rejoinder. Vaccine 2017. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Libr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Pietrantonj, C.D. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Rivetti, A.; Pietrantonj, C.D.; Demicheli, V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Reichert, T.A.; Viboud, C.; Blackwelder, W.C.; Taylor, R.J.; Miller, M.A. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch. Int. Med. 2005, 165, 265–272. [Google Scholar]
- Bar-Zeev, N.; Kapanda, L.; Tate, J.E.; Jere, K.C.; Iturriza-Gomara, M.; Nakagomi, O.; Mwansambo, C.; Costello, A.; Parashar, U.D.; Heyderman, R.S.; et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study. Lancet Infect. Dis. 2015, 15, 422–428. [Google Scholar] [CrossRef]
- Lopman, B.A.; Pitzer, V.E. Waxing Understanding of Waning Immunity. J. Infect. Dis. 2018, 217, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.G.M.; Gordon, S.B.; Lalloo, D.G. Clinical trials: The mathematics of falling vaccine efficacy with rising disease incidence. Vaccine 2016, 34, 3007. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, J.J.; Hernán, M.A.; Walensky, R.P.; Lipsitch, M. Apparent Declining Efficacy in Randomized Trials: Examples of the RV144 HIV Vaccine and CAPRISA 004 Microbicide Trials. AIDS 2012, 26, 123. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Sadarangani, S.; Jiang, L.; Wilder-Smith, A.; Chen, M.I. The Duration of Influenza Vaccine Effectiveness: A Systematic Review, Meta-analysis and Meta-regression of Test-Negative Design Case-control Studies. J. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cowling, B.J.; Kelly, H.; Sullivan, S.G. Estimating Influenza Vaccine Effectiveness in the Test-negative Design Using Alternative Control Groups—A Systematic Review and Meta-analysis. Am. J. Epidemiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Davenport, F.M.; Hennessy, A.V. A serologic recapitulation of past experiences with influenza A; Antibody response to monovalent vaccine. J. Exp. Med. 1956, 104, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Davenport, F.M.; Hennessy, A.V. Predetermination by infection and by vaccination of antibody response to influenza virus vaccines. J. Exp. Med. 1957, 106, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, L.; Ye, Z.; Li, X.; Plant, E.P.; Zoueva, O.; Zhao, Y.; Jing, X.; Lin, Z.; Kawano, T.; et al. Differential effects of prior influenza exposures on H3N2 cross-reactivity of human postvaccination sera. Clin. Infect. Dis. 2017, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.F.; Huang, Y.; Kaur, K.; Popova, L.I.; Ho, I.Y.; Pauli, N.T.; Dunand, C.J.H.; Taylor, W.M.; Lim, S.; Huang, M.; et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015, 7, 316ra192. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Smith, K.; Miller, J.; Langley, W.A.; Kokko, K.; Larsen, C.; Zheng, N.Y.; Mays, I.; Garman, L.; Helms, C.; et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453, 667. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Koutsonanos, D.; Li, G.M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, jem-20101352. [Google Scholar] [CrossRef]
- Miller, M.S.; Gardner, T.J.; Krammer, F.; Aguado, L.C.; Tortorella, D.; Basler, C.F.; Palese, P. Neutralizing antibodies against previously encountered influenza virus strains increase over time: A longitudinal analysis. Sci. Transl. Med. 2013, 5, 198ra107. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Riley, S.; Read, J.M.; Wang, S.; Zhu, H.; Smith, G.J.; Guan, Y.; Jiang, C.Q.; Cummings, D.A. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog. 2012, 8, e1002802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Q.; Wohlbold, T.J.; Zheng, N.Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Myers, J.L.; Bostick, D.L.; Sullivan, C.B.; Madara, J.; Linderman, S.L.; Liu, Q.; Carter, D.M.; Wrammert, J.; Esposito, S.; et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J. Exp. Med. 2013, jem-20130212. [Google Scholar] [CrossRef] [PubMed]
- Fonville, J.M.; Wilks, S.; James, S.L.; Fox, A.; Ventresca, M.; Aban, M.; Xue, L.; Jones, T.; Le, N.; Pham, Q.; et al. Antibody landscapes after influenza virus infection or vaccination. Science 2014, 346, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Lessler, J.; Read, J.M.; Zhu, H.; Jiang, C.Q.; Guan, Y.; Cummings, D.A.; Riley, S. Estimating the life course of influenza A (H3N2) antibody responses from cross-sectional data. PLoS Biol. 2015, 13, e1002082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linderman, S.L.; Hensley, S.E. Antibodies with ‘original antigenic sin’ properties are valuable components of secondary immune responses to influenza viruses. PLoS Pathog. 2016, 12, e1005806. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Davis, W.G.; Sambhara, S.; Jacob, J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 13751–13756. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.; Hensley, S.E. Immune history and influenza virus susceptibility. Curr. Opin. Virol. 2017, 22, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Ambrose, M.; Worobey, M.; Lloyd-Smith, J.O. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, A.; Acosta, E.; Hallman, S.; Bourbeau, R.; Dillon, L.Y.; Ouellette, N.; Earn, D.J.; Herring, D.A.; Inwood, K.; Madrenas, J.; et al. Pandemic Paradox: Early Life H2N2 Pandemic Influenza Infection Enhanced Susceptibility to Death during the 2009 H1N1 Pandemic. mBio 2018, 9, e02091-17. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Forrest, S.; Ackley, D.H.; Perelson, A.S. Variable efficacy of repeated annual influenza vaccination. Proc. Natl. Acad. Sci. USA 1999, 96, 14001–14006. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H.; et al. Serial vaccination and the antigenic distance hypothesis: Effects on influenza vaccine effectiveness during A (H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J. Infect. Dis. 2017, 215, 1059–1099. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.; Gouma, S.; Parkhouse, K.; Chambers, B.S.; Ertl, H.C.; Schmader, K.E.; Halpin, R.A.; Lin, X.; Stockwell, T.B.; Das, S.R.; et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012–2013. Clin. Infect. Dis. 2018, ciy097. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Zimmerman, R.K.; Gubareva, L.V.; Garten, R.J.; Chung, J.R.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Ohmit, S.E.; et al. Enhanced genetic characterization of influenza A (H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J. Infect. Dis. 2016, 214, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Linderman, S.L.; Chambers, B.S.; Zost, S.J.; Parkhouse, K.; Li, Y.; Herrmann, C.; Ellebedy, A.H.; Carter, D.M.; Andrews, S.F.; Zheng, N.Y.; et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proc. Natl. Acad. Sci. USA 2014, 111, 15798–15803. [Google Scholar] [CrossRef] [PubMed]
- Ndifon, W. A simple mechanistic explanation for original antigenic sin and its alleviation by adjuvants. J. R. Soc. Interface 2015, 12, 20150627. [Google Scholar] [CrossRef] [PubMed]
- Van der Most, R.G.; Roman, F.P.; Innis, B.; Hanon, E.; Vaughn, D.W.; Gillard, P.; Walravens, K.; Wettendorff, M. Seeking help: B cells adapting to flu variability. Sci. Transl. Med. 2014, 6, 246ps8. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.R.; Watanabe, A.; Kuraoka, M.; Do, K.T.; McGee, C.E.; Sempowski, G.D.; Kepler, T.B.; Schmidt, A.G.; Kelsoe, G.; Harrison, S.C. Memory B Cells that Cross-React with Group 1 and Group 2 Influenza A Viruses Are Abundant in Adult Human Repertoires. Immunity 2018, 48, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Choi, A.; Izikson, R.; Cox, M.M.; Palese, P.; Krammer, F. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. MBio 2016, 7, e01996-15. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Atmar, R.L.; Franco, L.M.; Quarles, J.M.; Wells, J.; Arden, N.; Niño, D.; Belmont, J.W. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis. 2013, 207, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.G.; Malosh, R.E.; Cheng, C.K.; Ohmit, S.E.; Martin, E.T.; Johnson, E.; Truscon, R.; Eichelberger, M.C.; Gubareva, L.V.; Fry, A.M.; et al. The Household Influenza Vaccine Effectiveness Study: Lack of Antibody Response and Protection Following Receipt of 2014–2015 Influenza Vaccine. Clin. Infect. Dis. 2017, 65, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Monto, A.S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis. 2011, 204, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.M.; Grill, D.E.; Oberg, A.L.; Tosh, P.K.; Ovsyannikova, I.G.; Poland, G.A. Profiles of influenza A/H1N1 vaccine response using hemagglutination-inhibition titers. Hum. Vaccines Immunother. 2015, 11, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.B.; Phadke, V.K.; Bednarczyk, R.A.; Chamberlain, A.T.; Brosseau, J.L.; Orenstein, W.A. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J. Infect. Dis. 2015, 213, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Chow, B.D.; VanWormer, J.J.; King, J.P.; Belongia, E.A. Effect of statin use on influenza vaccine effectiveness. J. Infect. Dis. 2016, 214, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.A.; Forshee, R.; Qiang, Y.; Wernecke, M.; Ferdinands, J.M.; Lu, Y.; Wei, Y.; Xu, W.; et al. Statin use and risks of influenza-related outcomes among older adults receiving standard-dose or high-dose influenza vaccines through Medicare during 2010–2015. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Jojic, V.; Sharma, S.; Shen-Orr, S.S.; Angel, C.J.; Onengut-Gumuscu, S.; Kidd, B.A.; Maecker, H.T.; Concannon, P.; Dekker, C.L.; et al. Cytomegalovirus infection enhances the immune response to influenza. Sci. Transl. Med. 2015, 7, 281ra43. [Google Scholar] [CrossRef] [PubMed]
- Trzonkowski, P.; Myśliwska, J.; Szmit, E.; Wieckiewicz, J.; Łukaszuk, K.; Brydak, L.B.; Machała, M.; Myśliwski, A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—An impact of immunosenescence. Vaccine 2003, 21, 3826–3836. [Google Scholar] [CrossRef]
- Klein, S.L.; Pekosz, A. Sex-based biology and the rational design of influenza vaccination strategies. J. Infect. Dis. 2014, 209, S114–S119. [Google Scholar] [CrossRef] [PubMed]
- HIPC-CHI Signatures Project Team; HIPC-I Consortium. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Wen, F.T.; Malani, A.; Cobey, S. Vaccination and the evolution of seasonal influenza. bioRxiv 2017, 162545. [Google Scholar] [CrossRef]
- Cai, F.Y.; Fussell, T.; Cobey, S.E.; Lipsitch, M. Use of an individual-based model of pneumococcal carriage for planning a randomized trial of a vaccine. bioRxiv 2018, 258871. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Gubbay, J.B.; Dickinson, J.A.; Fonseca, K.; Charest, H.; Bastien, N.; et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: Cross-season and cross-lineage protection with unchanged vaccine. J. Infect. Dis. 2014, 210, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Thompson, M.G.; Petrie, J.G.; Thaker, S.N.; Jackson, M.L.; Belongia, E.A.; Zimmerman, R.K.; Gaglani, M.; Lamerato, L.; Spencer, S.M.; et al. Influenza vaccine effectiveness in the 2011–2012 season: Protection against each circulating virus and the effect of prior vaccination on estimates. Clin. Infect. Dis. 2013, 58, 319–327. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Kieke, B.A.; Gaglani, M.; Murthy, K.; Piedra, P.A.; Zimmerman, R.K.; Nowalk, M.P.; Raviotta, J.M.; et al. Influenza vaccine effectiveness in the United States during 2012–2013: Variable protection by age and virus type. J. Infect. Dis. 2014, 211, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Gaglani, M.; Pruszynski, J.; Murthy, K.; Clipper, L.; Robertson, A.; Reis, M.; Chung, J.R.; Piedra, P.A.; Avadhanula, V.; Nowalk, M.P.; et al. Influenza vaccine effectiveness against 2009 pandemic influenza A (H1N1) virus differed by vaccine type during 2013–2014 in the United States. J. Infect. Dis. 2016, 213, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Johnson, E.; Truscon, R.; Aaron, B.; Martens, C.; Cheng, C.; Fry, A.M.; Monto, A.S. Substantial influenza vaccine effectiveness in households with children during the 2013–2014 influenza season, when 2009 pandemic influenza A (H1N1) virus predominated. J. Infect. Dis. 2015, 213, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.; Fonseca, K.; Charest, H.; Krajden, M.; et al. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013–2014 influenza season. J. Infect. Dis. 2015, 212, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.K.; Nowalk, M.P.; Chung, J.; Jackson, M.L.; Jackson, L.A.; Petrie, J.G.; Monto, A.S.; McLean, H.Q.; Belongia, E.A.; Gaglani, M.; et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin. Infect. Dis. 2016, ciw635. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.G.; Ohmit, S.E.; Cheng, C.K.; Martin, E.T.; Malosh, R.E.; Lauring, A.S.; Lamerato, L.E.; Reyes, K.C.; Flannery, B.; Ferdinands, J.M.; et al. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin. Infect. Dis. 2016, 63, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewnard, J.A.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines 2018, 6, 28. https://doi.org/10.3390/vaccines6020028
Lewnard JA, Cobey S. Immune History and Influenza Vaccine Effectiveness. Vaccines. 2018; 6(2):28. https://doi.org/10.3390/vaccines6020028
Chicago/Turabian StyleLewnard, Joseph A., and Sarah Cobey. 2018. "Immune History and Influenza Vaccine Effectiveness" Vaccines 6, no. 2: 28. https://doi.org/10.3390/vaccines6020028





