Feline Immunodeficiency Virus (FIV) Neutralization: A Review
Abstract
:1. Introduction
2. Measuring Neutralizing Antibody
2.1. Assays for HIV Neutralizing Antibodies
2.2. Assays for FIV Neutralizing Antibodies
2.3. Optimization of the CLL-CD134-Based Assay for FIV Neutralizing Antibody
3. FIV Neutralizing Antibodies
3.1. Time Course of Neutralizing Antibody Production in FIV Infection
3.2. Prevalence of Neutralizing Antibodies in FIV Infected Cats
3.3. Broadly Neutralizing Antibodies
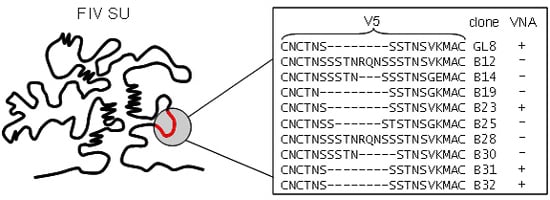
3.4. Determinants of FIV Neutralization
3.5. Neutralizing Antibodies Targeting the Membrane-Proximal External Region of FIV
3.6. Induction of FIV Neutralizing Antibodies by Vaccination
Acknowledgments
Conflict of Interest
References and Notes
- Klatzmann, D.; Champagne, E.; Chamaret, S.; Gruest, J.; Guetard, D.; Hercend, T.; Gluckman, J.C.; Montagnier, L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 1984, 312, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Maddon, P.J.; Dalgleish, A.G.; McDougal, J.S.; Clapham, P.R.; Weiss, R.A.; Axel, R. The T4 gene encodes the acquired immune deficiency syndrome virus receptor and is expressed in the immune system and the brain. Cell 1986, 43, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, A.G.; Beverley, P.C.; Clapham, P.R.; Crawford, D.H.; Greaves, M.F.; Weiss, R.A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 1984, 312, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Miyazawa, T.; Ikeda, Y.; McMonagle, E.L.; Haining, H.; Akashi, H.; Takeuchi, Y.; Hosie, M.J.; Willett, B.J. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 2004, 303, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. HIV-1 entry cofactor: Functional cDNA cloning of a seven transmembrane, G protein-coupled receptor. Science 1996, 272, 872–877. [Google Scholar] [CrossRef]
- Alkhatib, G.; Combadiere, C.; Broder, C.C.; Feng, Y.; Kennedy, P.E.; Murphy, P.M.; Berger, E.A. CC CKR5: A RANTES, MIP-1a, MIP-1b receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996, 272, 1955–1958. [Google Scholar] [CrossRef]
- Deng, H.; Liu, R.; Ellmeir, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Di Marzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 6584, 661–666. [Google Scholar] [CrossRef]
- Doranz, B.J.; Rucker, J.; Yi, Y.; Smyth, R.J.; Samson, M.; Peiper, S.C.; Parmentier, M.; Collman, R.G.; Doms, R.W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion co-factors. Cell 1996, 85, 1149–1158. [Google Scholar] [CrossRef]
- Dragic, T.; Litwin, T.; allaway, G.P.; Martin, S.R.; Huang, Y.; Nagashima, K.A.; Cayanan, C.; Maddon, P.J.; Koup, R.A.; Moore, J.P.; et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR5. Nature 1996, 6584, 667–673. [Google Scholar] [CrossRef]
- Willett, B.J.; Adema, K.A.; Heveker, N.; Brelot, A.; Picard, L.; Alizon, M.; Turner, J.D.; Hoxie, J.A.; Peiper, S.; Neil, J.C.; et al. The second extracellular loop of CXCR4 determines its function as a receptor for feline immunodeficiency virus. J. Virol. 1998, 72, 6475–6481. [Google Scholar] [CrossRef]
- Hosie, M.J.; Broere, N.; Hesselgesser, J.; Turner, J.D.; Hoxie, J.A.; Neil, J.C.; Willett, B.J. Modulation of feline immunodeficiency virus infection by stromal cell-derived factor (SDF-1). J. Virol. 1998, 72, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Picard, L.; Hosie, M.J.; Turner, J.D.; Adema, K.; Clapham, P.R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 1997, 71, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Hosie, M.J.; Neil, J.C.; Turner, J.D.; Hoxie, J.A. Common mechanism of infection by lentiviruses. Nature 1997, 385, 587. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Pancino, G.; Merat, R.; Leste-Lasserre, T.; Moraillon, A.; Schneider-Mergener, J.; Alizon, M.; Sonigo, P.; Heveker, N. Shared usage of the chemokine receptor CXCR4 by primary and laboratory-adapted strains of feline immunodeficiency virus. J. Virol. 1999, 73, 3661–3671. [Google Scholar] [CrossRef] [PubMed]
- Egberink, H.F.; De Clercq, E.; Van Vliet, A.L.; Balzarini, J.; Bridger, G.J.; Henson, G.; Horzinek, M.C.; Schols, D. Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication. J. Virol. 1999, 73, 6346–6352. [Google Scholar] [CrossRef]
- Stamatatos, L.; Morris, L.; Burton, D.R.; Mascola, J.R. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat. Med. 2009, 15, 866–870. [Google Scholar] [CrossRef]
- Pantophlet, R.; Burton, D.R. GP120: Target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 2006, 24, 739–769. [Google Scholar] [CrossRef]
- Weiss, R.A.; Clapham, P.R.; Cheingsong-Popov, R.; Dalgleish, A.G.; Carne, C.A.; Weller, I.V.D.; Tedder, R.S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature 1985, 316, 69. [Google Scholar] [CrossRef]
- Robert-Guroff, M.; Brown, M.; Gallo, R.C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature 1985, 316, 72–74. [Google Scholar] [CrossRef]
- Clapham, P.; Nagy, K.; Weiss, R.A. Pseudotypes of human T-cell leukemia virus types 1 and 2: neutralization by patients’ sera. Proc. Natl. Acad. Sci. U. S. A. 1984, 81, 2886–2889. [Google Scholar] [CrossRef]
- Brown, B.K.; Darden, J.M.; Tovanabutra, S.; Oblander, T.; Frost, J.; Sanders-Buell, E.; de Souza, M.S.; Birx, D.L.; McCutchan, F.E.; Polonis, V.R. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J. Virol. 2005, 79, 6089–6101. [Google Scholar] [CrossRef] [PubMed]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; D’Souza, P.; Gilbert, P.; Hahn, B.H.; Haigwood, N.L.; Morris, L.; Petropoulos, C.J.; Polonis, V.R.; Sarzotti, M.; Montefiori, D.C. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 2005, 79, 10103–10107. [Google Scholar] [CrossRef]
- Li, M.; Gao, F.; Mascola, J.R.; Stamatatos, L.; Polonis, V.R.; Koutsoukos, M.; Voss, G.; Goepfert, P.; Gilbert, P.; Greene, K.M.; et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005, 79, 10108–10125. [Google Scholar] [CrossRef]
- Brown, B.K.; Wieczorek, L.; Sanders-Buell, E.; Rosa, B.A.; Robb, M.L.; Birx, D.L.; Michael, N.L.; McCutchan, F.E.; Polonis, V.R. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 2008, 375, 529–538. [Google Scholar] [CrossRef]
- Binley, J.M.; Wrin, T.; Korber, B.; Zwick, M.B.; Wang, M.; Chappey, C.; Stiegler, G.; Kunert, R.; Zolla-Pazner, S.; Katinger, H.; et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 2004, 78, 13232–13252. [Google Scholar] [CrossRef]
- Polonis, V.R.; Brown, B.K.; Rosa, B.A.; Zolla-Pazner, S.; Dimitrov, D.S.; Zhang, M.Y.; Barnett, S.W.; Ruprecht, R.M.; Scarlatti, G.; Fenyo, E.M.; et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 2008, 375, 315–320. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Ho, E.W.; Brown, M.L.; Yamamoto, J.K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987, 235, 790–793. [Google Scholar] [CrossRef]
- de Parseval, A.; Chatterji, U.; Sun, P.; Elder, J.H. Feline immunodeficiency virus targets activated CD4+ T cells by using CD134 as a binding receptor. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 13044–13049. [Google Scholar] [CrossRef]
- Fevereiro, M.; Roneker, C.; Laufs, A.; Tavares, L.; de Noronha, F. Characterisation of two monoclonal antibodies against feline immunodeficiency virus gag gene products and their application in an assay to evaluate neutralising antibody activity. J. Gen. Virol. 1991, 72, 617–622. [Google Scholar] [CrossRef]
- Osborne, R.; Rigby, M.; Siebelink, K.; Neil, J.C.; Jarrett, O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J. Gen. Virol. 1994, 75, 3641–3645. [Google Scholar] [CrossRef] [PubMed]
- Tozzini, F.; Matteucci, D.; Bandecchi, P.; Baldinotti, F.; Poli, A.; Pistello, M.; Siebelink, K.H.J.; Ceccherini-Nelli, L.; Bendinelli, M. Simple in vitro methods for titrating feline immunodeficiency virus (FIV) and FIV neutralising antibodies. J. Virol. Meth. 1992, 37, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Fossati, I.; Moraillon, A.; Castelot, S.; Sonigo, P.; Pancino, G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J. Gen. Virol. 1996, 77, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Samman, A.; Logan, N.; McMonagle, E.L.; Ishida, T.; Mochizuki, M.; Willett, B.J.; Hosie, M.J. Neutralization of feline immunodeficiency virus by antibodies targeting the V5 loop of Env. J. Gen. Virol. 2010, 91, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.M.; Furuya, T.; Itagaki, S.; Tohya, Y.; Takahashi, E.; Mikami, T. Establishment of a feline T-lymphoblastoid cell line highly sensitive for replication of feline immunodeficiency virus. Arch. Virol. 1989, 108, 131–135. [Google Scholar] [CrossRef]
- Hosie, M.J.; Klein, D.; Binley, J.M.; Dunsford, T.; Jarrett, O.; Neil, J.; Knapp, E.; Giannecchini, S.; Matteucci, D.; Bendinelli, M.; et al. Vaccination with an inactivated virulent feline immunodeficiency virus engineered to express high levels of Env. J. Virol. 2005, 79, 1954–1957. [Google Scholar] [CrossRef]
- Willett, B.J.; McMonagle, E.L.; Logan, N.; Samman, A.; Hosie, M.J. A single site for N-linked glycosylation in the envelope glycoprotein of feline immunodeficiency virus modulates the virus-receptor interaction. Retrovirology 2008, 5, 77. [Google Scholar] [CrossRef]
- Hosie, M.J.; Flynn, N.; Rigby, M.A.; Cannon, C.; Dunsford, T.; Mackay, N.; Argyle, D.; Willett, B.J.; Miyazawa, T.; Onions, D.E.; et al. DNA Vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J. Virol. 1998, 72, 7310–7319. [Google Scholar] [CrossRef]
- Sierra, S.; Kupfer, B.; Kaiser, R. Basics of the virology of HIV-1 and its replication. J. Clin. Virol. 2005, 34, 233–244. [Google Scholar] [CrossRef]
- Pantaleo, G.; Graziosi, C.; Demarest, J.F.; Butini, L.; Montroni, M.; Fox, C.H.; Orenstein, J.M.; Kotler, D.P.; Fauci, A.S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993, 362, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Richman, D.D.; Wrin, T.; Little, S.J.; Petropoulos, C.J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 4144–4149. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Decker, J.M.; Johnson, R.W.; Bibollet-Ruche, F.; Wei, X.; Mulenga, J.; Allen, S.; Hunter, E.; Hahn, B.H.; Shaw, G.M.; et al. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J. Virol. 2006, 80, 5211–5218. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.L.; Gray, E.S.; Choge, I.A.; Ranchobe, N.; Mlisana, K.; Abdool Karim, S.S.; Williamson, C.; Morris, L. The c3-v4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J. Virol. 2008, 82, 1860–1869. [Google Scholar] [CrossRef]
- Doria-Rose, N.A.; Klein, R.M.; Daniels, M.G.; O’Dell, S.; Nason, M.; Lapedes, A.; Bhattacharya, T.; Migueles, S.A.; Wyatt, R.T.; Korber, B.T.; et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 2010, 84, 1631–1636. [Google Scholar] [CrossRef]
- Sather, D.N.; Armann, J.; Ching, L.K.; Mavrantoni, A.; Sellhorn, G.; Caldwell, Z.; Yu, X.; Wood, B.; Self, S.; Kalams, S.; et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 2009, 83, 757–769. [Google Scholar] [CrossRef]
- Simek, M.D.; Rida, W.; Priddy, F.H.; Pung, P.; Carrow, E.; Laufer, D.S.; Lehrman, J.K.; Boaz, M.; Tarragona-Fiol, T.; Miiro, G.; et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009, 83, 7337–7348. [Google Scholar] [CrossRef]
- Euler, Z.; van Gils, M.J.; Bunnik, E.M.; Phung, P.; Schweighardt, B.; Wrin, T.; Schuitemaker, H. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J. Infect. Dis. 2010, 201, 1045–1053. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.F.; Siebelink, K.H.; Broos, H.; Drost, G.A.; Weijer, K.; van, H.R.; Osterhaus, A.D. gag- and env-specific serum antibodies in cats after natural and experimental infection with feline immunodeficiency virus. Vet. Microbiol. 1994, 39, 153–165. [Google Scholar] [CrossRef]
- Hosie, M.J.; Willett, B.J.; Klein, D.; Dunsford, T.H.; Cannon, C.; Shimojima, M.; Neil, J.; Jarrett, O. Evolution of replication efficiency following infection with a molecularly cloned feline immunodeficiency virus of low virulence. J. Virol. 2002, 76, 6062–6072. [Google Scholar] [CrossRef]
- Kraase, M.; Sloan, R.; Klein, D.; Logan, N.; McMonagle, L.; Biek, R.; Willett, B.J.; Hosie, M.J. Feline immunodeficiency virus env gene evolution in experimentally infected cats. Vet. Immunol. Immunopathol. 2010, 134, 96–106. [Google Scholar] [CrossRef]
- Willett, B.J.; Kraase, M.; Logan, N.; McMonagle, E.L.; Samman, A.; Hosie, M.J. Modulation of the virus-receptor interaction by mutations in the V5 loop of feline immunodeficiency virus (FIV) following in vivo escape from neutralising antibody. Retrovirology 2010, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Courchamp, F.; Pontier, D. Feline immunodeficiency virus: An epidemiological review. C. R. Acad. Sci. III 1994, 317, 1123–1134. [Google Scholar] [PubMed]
- Natoli, E.; Say, L.; Cafazzo, S.; Bonanni, R.; Schmid, M.; Pontier, D. Bold attitude makes male urban feral domestic cats more vulnerable to Feline Immunodeficiency Virus. Neurosci. Biobehav. Rev. 2005, 29, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Cao, Y.; Leu, J.; Qin, L.; Korber, B.; Ho, D.D. Inter- and intraclade neutralisaton of human immunodeficiency virus type 1: Genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 1996, 70, 427–444. [Google Scholar] [CrossRef]
- Beirnaert, E.; Nyambi, P.; Willems, B.; Heyndrickx, L.; Colebunders, R.; Janssens, W.; van der Groen, G. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 2000, 62, 14–24. [Google Scholar] [CrossRef]
- Li, Y.; Migueles, S.A.; Welcher, B.; Svehla, K.; Phogat, A.; Louder, M.K.; Wu, X.; Shaw, G.M.; Connors, M.; Wyatt, R.T.; et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 2007, 13, 1032–1034. [Google Scholar] [CrossRef]
- Binley, J.M.; Lybarger, E.A.; Crooks, E.T.; Seaman, M.S.; Gray, E.; Davis, K.L.; Decker, J.M.; Wycuff, D.; Harris, L.; Hawkins, N.; et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 2008, 82, 11651–11668. [Google Scholar] [CrossRef]
- Samman, A.; McMonagle, E.L.; Logan, N.; Willett, B.J.; Biek, R.; Hosie, M.J. Phylogenetic characterisation of naturally occurring feline immunodeficiency virus in the United Kingdom. Vet. Microbiol. 2011, 150, 239–247. [Google Scholar] [CrossRef]
- Medina-Ramirez, M.; Sanchez-Merino, V.; Sanchez-Palomino, S.; Merino-Mansilla, A.; Ferreira, C.B.; Perez, I.; Gonzalez, N.; Alvarez, A.; Alcocer-Gonzalez, J.M.; Garcia, F.; et al. Broadly cross-neutralizing antibodies in HIV-1 patients with undetectable viremia. J. Virol. 2011, 85, 5804–5813. [Google Scholar] [CrossRef]
- Lombardi, S.; Garzelli, C.; La Rosa, C.; Zaccaro, L.; Specter, S.; Malvaldi, G.; Tozzini, F.; Esposito, F.; Bendinelli, M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J. Virol. 1993, 67, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.; Garzelli, C.; Pistello, M.; Massi, C.; Matteucci, D.; Baldinotti, F.; Cammaroto, G.; Da Prato, L.; Bandecchi, P.; Tozzini, F.; et al. A neutralising antibody-inducing peptide of the V3 domain of feline immunodeficiency virus envelope glycoprotein does not induce protective immunity. J. Virol. 1994, 68, 8374–8379. [Google Scholar] [CrossRef]
- de, P.A.; Grant, C.K.; Sastry, K.J.; Elder, J.H. Sequential CD134-CXCR4 interactions in feline immunodeficiency virus (FIV): soluble CD134 activates FIV Env for CXCR4-dependent entry and reveals a cryptic neutralization epitope. J. Virol. 2006, 80, 3088–3091. [Google Scholar]
- Giannecchini, S.; Del Mauro, D.; Matteucci, D.; Bendinelli, M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: reevaluation of neutralizing antobody levels elicited by a protective and a nonprotective vaccine after removal of antisubstrate cell antibodies. J. Virol. 2001, 75, 4424–4429. [Google Scholar] [CrossRef] [PubMed]
- Pistello, M.; Matteucci, D.; Giannecchini, S.; Bonci, F.; Sichi, O.; Presciuttini, S.; Bendinelli, M. Evolution of two amino acid positions governing broad neutralization resistance in a strain of feline immunodeficiency virus over 7 years of persistence in cats. Clin. Diagn. Lab. Immunol. 2003, 10, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Siebelink, K.H.J.; Rimmelzwaan, G.F.; Bosch, M.L.; Meloen, R.H.; Osterhaus, A.D.M.E. A single amino acid substitution in hypervariable region 5 of the envelope protein of feline immunodeficiency virus allows escape from virus neutralisation. J. Virol. 1993, 67, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, M.; Pistello, M.; Del Mauro, D.; Cammarota, G.; Maggi, F.; Leonildi, A.; Giannecchini, S.; Bergamini, C.; Matteucci, D. During readaptation in vivo, a tissue culture-adapted strain of feline immunodeficiency virus reverts to broad neutralization resistance at different times in individual hosts but through changes at the same position of the surface glycoprotein. J. Virol. 2001, 75, 4584–4593. [Google Scholar] [CrossRef]
- Siebelink, K.H.J.; Huisman, W.; Karlas, J.A.; Rimmelzwaan, G.F.; Bosch, M.L.; Osterhaus, A.D.M.E. Neutralization of feline immunodeficiency virus by polyclonal feline antibody: Simultaneous involvement of hypervariable regions 4 and 5 of the surface glycoprotein. J. Virol. 1995, 69, 5124–5127. [Google Scholar] [CrossRef]
- Giannecchini, S.; Isola, P.; Sichi, O.; Matteucci, D.; Pistello, M.; Zaccaro, L.; Del Mauro, D.; Bendinelli, M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: Failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts. J. Virol. 2002, 76, 6882–6892. [Google Scholar] [CrossRef]
- Bernstein, H.B.; Tucker, S.P.; Hunter, E.; Schutzbach, J.S.; Compans, R.W. Human immunodeficiency virus type 1 envelope glycoprotein is modified by O-linked oligosaccharides. J. Virol. 1994, 68, 463–468. [Google Scholar] [CrossRef]
- Huang, X.; Barchi, J.J., Jr.; Lung, F.D.; Roller, P.P.; Nara, P.L.; Muschik, J.; Garrity, R.R. Glycosylation affects both the three-dimensional structure and antibody binding properties of the HIV-1IIIB GP120 peptide RP135. Biochemistry 1997, 36, 10846–10856. [Google Scholar] [CrossRef] [PubMed]
- Bunnik, E.M.; Pisas, L.; van Nuenen, A.C.; Schuitemaker, H. Autologous neutralizing humoral immunity and evolution of the viral envelope in the course of subtype B human immunodeficiency virus type 1 infection. J. Virol. 2008, 82, 7932–7941. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Gnanakaran, S.; Decker, J.M.; Bibollet-Ruche, F.; Taylor, J.; Sfakianos, J.N.; Mokili, J.L.; Muldoon, M.; Mulenga, J.; Allen, S.; et al. Unique mutational patterns in the envelope alpha 2 amphipathic helix and acquisition of length in gp120 hypervariable domains are associated with resistance to autologous neutralization of subtype C human immunodeficiency virus type 1. J. Virol. 2007, 81, 5658–5668. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Bibollet-Ruche, F.; Mulenga, J.; Allen, S.; Blackwell, J.L.; Derdeyn, C.A. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J. Virol. 2007, 81, 1350–1359. [Google Scholar] [CrossRef]
- Sagar, M.; Wu, X.; Lee, S.; Overbaugh, J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 2006, 80, 9586–9598. [Google Scholar] [CrossRef]
- Wei, X.; Decker, J.M.; Wang, S.; Hui, H.; Kappes, J.C.; Wu, X.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Kilby, J.M.; Saag, M.S.; et al. Antibody neutralization and escape by HIV-1. Nature 2003, 422, 307–312. [Google Scholar] [CrossRef]
- Bunnik, E.M.; Euler, Z.; Welkers, M.R.; Boeser-Nunnink, B.D.; Grijsen, M.L.; Prins, J.M.; Schuitemaker, H. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat. Med. 2010, 16, 995–997. [Google Scholar] [CrossRef]
- Motokawa, K.; Hohdatsu, T.; Imori, A.; Arai, S.; Koyama, H. Mutations in feline immunodeficiency (FIV) virus envelope gene V3-V5 regions in FIV-infected cats. Vet. Microbiol. 2005, 106, 33–40. [Google Scholar] [CrossRef]
- Ikeda, Y.; Miyazawa, T.; Nishimura, Y.; Nakamura, K.; Tohya, Y.; Mikami, T. High genetic stability of TM1 and TM2 strains of subtype B feline immunodeficiency virus in long-term infection. J. Vet. Med. Sci. 2004, 66, 287–289. [Google Scholar] [CrossRef]
- Huisman, W.; Schrauwen, E.J.; Rimmelzwaan, G.F.; Osterhaus, A.D. Intrahost evolution of envelope glycoprotein and OrfA sequences after experimental infection of cats with a molecular clone and a biological isolate of feline immunodeficiency virus. Virus Res. 2008, 137, 24–32. [Google Scholar] [CrossRef]
- Carpenter, S.; Vaughn, E.M.; Yang, J.; Baccam, P.; Roth, J.A.; Wannemuehler, Y. Antigenic and genetic stability of bovine immunodeficiency virus during long-term persistence in cattle experimentally infected with the BIV(R29) isolate. J. Gen. Virol. 2000, 81, 1463–1472. [Google Scholar] [CrossRef]
- Eastman, D.; Piantadosi, A.; Wu, X.; Forthal, D.N.; Landucci, G.; Kimata, J.T.; Overbaugh, J. Heavily glycosylated, highly fit SIVMne variants continue to diversify and undergo selection after transmission to a new host and they elicit early antibody dependent cellular responses but delayed neutralizing antibody responses. Virol. J. 2008, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Zwick, M.B. The membrane-proximal external region of HIV-1 gp41: A vaccine target worth exploring. AIDS 2005, 19, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Giannecchini, S.; Di, F.A.; D’Ursi, A.M.; Matteucci, D.; Rovero, P.; Bendinelli, M. Antiviral activity and conformational features of an octapeptide derived from the membrane-proximal ectodomain of the feline immunodeficiency virus transmembrane glycoprotein. J. Virol. 2003, 77, 3724–3733. [Google Scholar] [CrossRef]
- Freer, G.; Giannecchini, S.; Tissot, A.; Bachmann, M.F.; Rovero, P.; Serres, P.F.; Bendinelli, M. Dissection of seroreactivity against the tryptophan-rich motif of the feline immunodeficiency virus transmembrane glycoprotein. Virology 2004, 322, 360–369. [Google Scholar] [CrossRef]
- Esposito, C.; D’Errico, G.; Armenante, M.R.; Giannecchini, S.; Bendinelli, M.; Rovero, P.; D’Ursi, A.M. Physicochemical characterization of a peptide deriving from the glycoprotein gp36 of the feline immunodeficiency virus and its lipoylated analogue in micellar systems. Biochim. Biophys. Acta 2006, 1758, 1653–1661. [Google Scholar] [CrossRef]
- Giannecchini, S.; D’Ursi, A.M.; Esposito, C.; Scrima, M.; Zabogli, E.; Freer, G.; Rovero, P.; Bendinelli, M. Antibodies generated in cats by a lipopeptide reproducing the membrane-proximal external region of the feline immunodeficiency virus transmembrane enhance virus infectivity. Clin. Vaccine Immunol. 2007, 14, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, D.; Mazzetti, P.; Baldinotti, F.; Zaccaro, L.; Bendinelli, M. The feline lymphoid cell line MBM and its use for feline immunodeficiency virus isolation and quantitation. Vet. Immunol. Immunopathol. 1995, 46, 71–82. [Google Scholar] [CrossRef]
- Huisman, W.; Schrauwen, E.J.; Pas, S.D.; Karlas, J.A.; Rimmelzwaan, G.F.; Osterhaus, A.D. Antibodies specific for hypervariable regions 3 to 5 of the feline immunodeficiency virus envelope glycoprotein are not solely responsible for vaccine-induced acceleration of challenge infection in cats. J. Gen. Virol. 2004, 85, 1833–1841. [Google Scholar] [CrossRef]
- Karlas, J.A.; Siebelink, K.H.; Peer, M.A.; Huisman, W.; Cuisinier, A.M.; Rimmelzwaan, G.F.; Osterhaus, A.D. Vaccination with experimental feline immunodeficiency virus vaccines, based on autologous infected cells, elicits enhancement of homologous challenge infection. J. Gen. Virol. 1999, 80, 761–765. [Google Scholar] [CrossRef]
- Karlas, J.A.; Siebelink, K.H.; Peer, M.A.; Huisman, W.; Rimmelzwaan, G.F.; Osterhaus, A.D. Accelerated viraemia in cats vaccinated with fixed autologous FIV- infected cells. Vet. Immunol. Immunopathol. 1998, 65, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Moraillon, A.; Baud, S.; Cuisinier, A.-M.; Sonigo, P.; Pancino, G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J. Virol. 1997, 71, 9640–9649. [Google Scholar] [CrossRef] [PubMed]
- Siebelink, K.H.J.; Tijhaar, E.; Huisman, R.C.; Huisman, W.; de Ronde, A.; Darby, I.H.; Francis, M.J.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Enhancement of feline immunodeficiency virus infection after immunisation with envelope glycoprotein subunit vaccines. J. Virol. 1995, 69, 3704–3711. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Hohdatsu, T.; Olmsted, R.A.; Pu, R.; Louie, H.; Zochlinski, H.A.; Acevedo, V.; Johnson, H.M.; Soulds, G.A.; Gardner, M.B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J. Virol. 1993, 67, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Okuda, T.; Ackley, C.D.; Louie, H.; Pembroke, E.; Zochlinski, H.; Munn, R.J.; Gardner, M.B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res. Hum. Retro. 1991, 7, 911–921. [Google Scholar] [CrossRef]
- Hosie, M.J.; Osborne, R.; Yamamoto, J.K.; Neil, J.C.; Jarrett, O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J. Virol. 1995, 69, 1253–1255. [Google Scholar] [CrossRef]
- Hosie, M.J.; Dunsford, T.H.; de Ronde, A.; Willett, B.J.; Cannon, C.A.; Neil, J.C.; Jarrett, O. Suppression of virus burden by immunisation with feline immunodeficiency virus Env protein. Vaccine 1996, 14, 405–411. [Google Scholar] [CrossRef]
- Flynn, J.N.; Keating, P.; Hosie, M.J.; Mackett, M.; Stephens, E.B.; Beatty, J.A.; Neil, J.C.; Jarrett, O. Env-specific CTL predominate in cats protected from FIV infection by vaccination. J. Immunol. 1996, 157, 3658–3665. [Google Scholar] [CrossRef]
- Flynn, J.N.; Beatty, J.A.; Cannon, C.A.; Stephens, E.B.; Hosie, M.J.; Neil, J.C.; Jarrett, O. Involvement of gag- and env- specific cytotoxic T lymphocytes in protective immunity to feline immunodeficiency virus. AIDS Res. Hum. Retro. 1995, 11, 1107–1113. [Google Scholar] [CrossRef]
- Pu, R.; Okada, S.; Little, E.R.; Xu, B.; Stoffs, W.V.; Yamamoto, J.K. Protection of neonatal kittens against feline immunodeficiency virus infection with passive maternal antiviral antibodies. AIDS 1997, 9, 235–242. [Google Scholar] [CrossRef]
- Pistello, M.; Matteucci, D.; Bonci, F.; Isola, P.; Mazzetti, P.; Zaccaro, L.; Merico, A.; Del, M.D.; Flynn, N.; Bendinelli, M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: protection from an intraclade challenge administered systemically or mucosally by an attenuated vaccine. J. Virol. 2003, 77, 10740–10750. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.Y.; Coleman, J.; Coisman, J.; Sato, E.; Tanabe, T.; Arai, M.; Yamamoto, J.K. Dual-subtype FIV vaccine (Fel-O-Vax((R)) FIV) protection against a heterologous subtype BFIV isolate. J. Fel. Med. Surg. 2005, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.; Coleman, J.; Omori, M.; Arai, M.; Hohdatsu, T.; Huang, C.; Tanabe, T.; Yamamoto, J.K. Dual-subtype FIV vaccine protects cats against in vivo swarms of both homologous and heterologous subtype FIV isolates. AIDS 2001, 15, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Hohdatsu, T.; Okada, S.; Motokawa, K.; Aizawa, C.; Yamamoto, J.K.; Koyama, H. Effect of dual-subtype vaccine against feline immunodeficiency virus infection. Vet. Microbiol. 1997, 58, 155–165. [Google Scholar] [CrossRef]



| Plasma | ||||
|---|---|---|---|---|
| Virus | Origin | Subtype | 178639 | 206394 |
| GL8 | UK | A | 99 | 95 |
| 180638 | UK | A | 99 | 90 |
| 171838 | UK | A | 99 | 99 |
| 180260 | UK | A | 96 | 96 |
| 180140 | UK | A | 90 | 97 |
| 178721 | UK | A | 99 | 97 |
| 179200 | UK | A | 83 | 97 |
| 206394 | UK | A | 83 | 93 |
| 0425 | UK | A | 99 | 96 |
| 0827 | UK | A | 99 | 95 |
| 1419 | UK | A | 95 | 96 |
| PPR | USA | A | 98 | 99 |
| B2542 | USA | B | 99 | 99 |
| KNG2 | Japan | B | 96 | 99 |
| TM2 | Japan | B | 99 | 99 |
| Pisa M2 | Italy | B | 93 | 100 |
| Leviano | Brasil | B | 90 | 100 |
| CPG41 | USA | C | 96 | 99 |
| Poose | Sri Lanka | - | 73 | 100 |
| LLV-B | Tanzania | - | 51 | 16 |
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosie, M.J.; Pajek, D.; Samman, A.; Willett, B.J. Feline Immunodeficiency Virus (FIV) Neutralization: A Review. Viruses 2011, 3, 1870-1890. https://doi.org/10.3390/v3101870
Hosie MJ, Pajek D, Samman A, Willett BJ. Feline Immunodeficiency Virus (FIV) Neutralization: A Review. Viruses. 2011; 3(10):1870-1890. https://doi.org/10.3390/v3101870
Chicago/Turabian StyleHosie, Margaret J., Daniela Pajek, Ayman Samman, and Brian J. Willett. 2011. "Feline Immunodeficiency Virus (FIV) Neutralization: A Review" Viruses 3, no. 10: 1870-1890. https://doi.org/10.3390/v3101870