Baltikinin: A New Myotropic Tryptophyllin-3 Peptide Isolated from the Skin Secretion of the Purple-Sided Leaf Frog, Phyllomedusa baltea
Abstract
:1. Introduction
2. Results
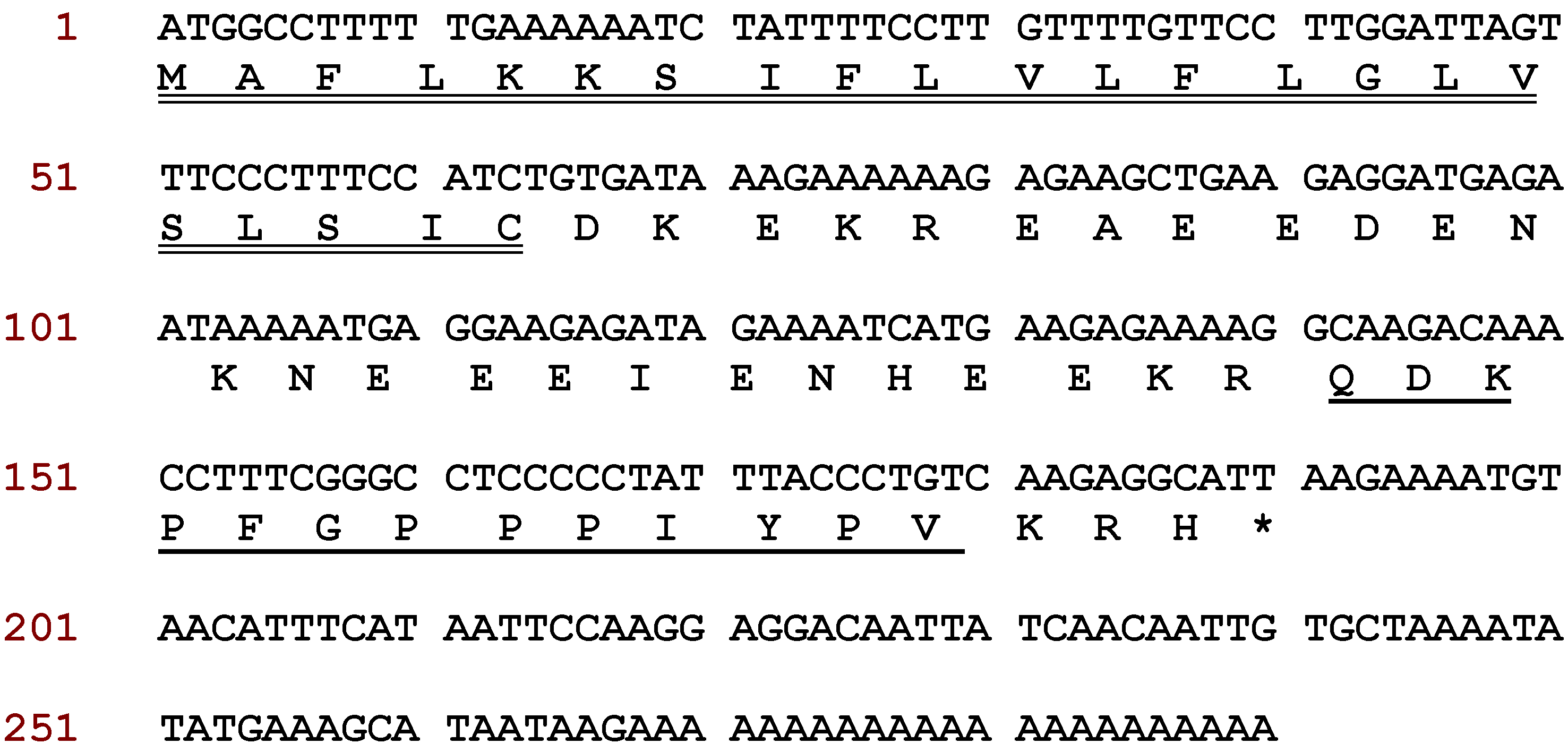
2.1. Molecular Cloning of Baltikinin Precursor-Encoding cDNA from a Skin Secretion-Derived cDNA Library of Phyllomedusa Baltea
2.2. Identification and Structural Analysis of Baltikinin
2.3. Peptide Synthesis
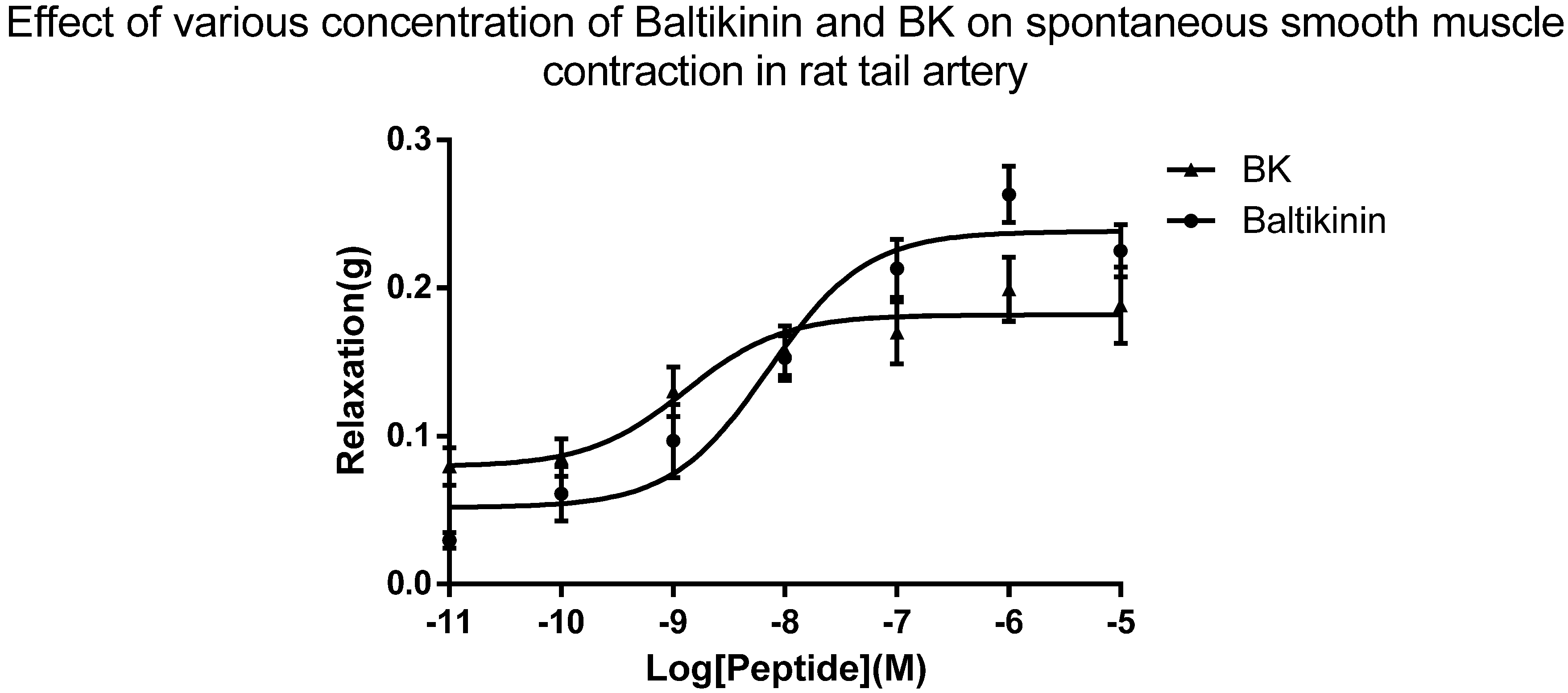
2.4. Arterial Smooth Muscle Pharmacology
3. Discussion
4. Materials and Methods
4.1. Skin Secretions
4.2. “Shotgun” Cloning of Preprobaltikinin Encoding cDNA
4.3. Identification and Structural Characterisation of Baltikinin from the Skin Secretion
4.4. Peptide Synthesis
4.5. Rat Tail Artery Smooth Muscle Bioassay
4.5.1. Tissue Preparation
4.5.2. Pharmacological Bioassay
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| HPLC | High performance liquid chromatography |
| cDNA | complmentary deoxyribonucleic acid |
| BLAST | Basic Local Alignment Search Tool |
| NCBI | National Centre for Biotechnological Information |
| MALDI-TOF | Matrix-assisted laser desorption/ionisation, time-of-flight |
| MS | mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| SEM | Standard error of the mean |
| TFA | Trifluoroacetic acid |
| PS | Peptide synthesiser |
| LC/MS/MS | Liquid chromatography coupled tandem mass spectrometry |
References
- Roelants, K.; Fry, B.G.; Ye, L.; Stijlemans, B.; Brys, L.; Kok, P.; Clynen, E.; Schoofs, L.; Cornelis, P.; Bossuyt, F. Origin and functional diversification of an amphibian defense peptide arsenal. PLoS Genet. 2013, 9, e1003662. [Google Scholar] [CrossRef] [PubMed]
- Pukala, T.L.; Bowie, J.H.; Maselli, V.M.; Musgrave, I.F.; Tyler, M.J. Host-defence peptides from the glandular secretions of amphibians: Structure and activity. Nat. Prod. Rep. 2006, 23, 368–393. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M. Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell. Mol. Life Sci. 2011, 68, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V. Bioactive Secretions of the Amphibian Integument. In Amphibian Biology; Heatwole, H., Barthalmus, G.T., Eds.; Surrey Beatty & Sons: Chipping Norton, Australia, 1994; Volume 1, pp. 178–350. [Google Scholar]
- Erspamer, V.; Falconieri Erspamer, G.; Cei, J.M. Active peptides in the skins of two hundred and thirty American amphibian species. Comp. Biochem. Physiol. C 1986, 85, 125–137. [Google Scholar] [CrossRef]
- Erspamer, V.; Melchiorri, P.; Falconieri Erspamer, G.; Montecucchi, P.C.; de Castiglione, R. Phyllomedusa skin: A huge factory and store-house of a variety of active peptides. Peptides 1985, 6, 7–12. [Google Scholar] [CrossRef]
- Calderon, L.D.; Silva, A.D.E.; Ciancaglini, P.; Stabeli, R.G. Antimicrobial peptides from Phyllomedusa frogs: From biomolecular diversity to potential nanotechnologic medical applications. Amino Acids 2011, 40, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Severini, C.; Improta, G.; Falconieri-Erspamer, G.; Salvadori, S.; Erspamer, V. The tachykinin peptide family. Pharmacol. Rev. 2002, 54, 285–322. [Google Scholar] [CrossRef] [PubMed]
- Falconieri Erspamer, G.; Severini, C.; Erspamer, V.; Melchiorri, P.; Delle Fave, G.; Nakajima, T. Parallel bioassay of 27 bombesin-like peptides on 9 smooth muscle preparations. Structure-activity relationships and bombesin receptor subtypes. Regul. Pept. 1988, 21, 1–11. [Google Scholar] [CrossRef]
- Shi, D.; Luo, Y.; Du, Q.; Wang, L.; Zhou, M.; Ma, J.; Li, R.; Chen, T.; Shaw, C. A novel bradykinin-related dodecapeptide (RVALPPGFTPLR) from the skin secretion of the fujian large-headed frog (Limnonectes fujianensis) exhibiting unusual structural and functional features. Toxins 2014, 6, 2886–2898. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xi, X.; Ge, L.; Yang, N.; Hou, X.; Ma, J.; Ma, C.; Wu, Y.; Guo, X.; Li, R.; et al. Bradykinin-related peptides (BRPs) from skin secretions of three genera of phyllomedusine leaf frogs and their comparative pharmacological effects on mammalian smooth muscles. Peptides 2014, 52, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, M.; Zhou, Z.; Chen, T.; Walker, B.; Shaw, C. Sauvatide—A novel amidated myotropic decapeptide from the skin secretion of the waxy monkey frog, Phyllomedusa sauvagei. Biochem. Biophys. Res. Commun. 2009, 383, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Orr, D.F.; O’Rourke, M.; McLynn, C.; Bjourson, A.J.; McClean, S.; Hirst, D.; Rao, P.; Shaw, C. Pachymedusa dacnicolor tryptophyllin-1: Structural characterization, pharmacological activity and cloning of precursor cDNA. Regul. Pept. 2004, 117, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, M.; Chen, W.; Lorimer, J.; Rao, P.; Walker, B.; Shaw, C. Cloning from tissue surrogates: Antimicrobial peptide (esculentin) cDNAs from the defensive skin secretions of Chinese ranid frogs. Genomics 2006, 87, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Farragher, S.; Bjourson, A.J.; Orr, D.F.; Rao, P.; Shaw, C. Granular gland transcriptomes in stimulated amphibian skin secretions. Biochem. J. 2003, 371, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, Y.; Chen, T.; Zhou, M.; Wang, L.; Shaw, C. Molecular cloning of a novel tryptophyllin peptide from the skin of the orange-legged monkey frog, Phyllomedusa hypochondrialis. Chem. Biol. Drug Des. 2014, 83, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, Y.; Chen, T.; Zhou, M.; Wang, L.; Shaw, C. Identification and functional analysis of a novel tryptophyllin peptide from the skin of the red-eye leaf frog, Agalychnis callidryas. Int. J. Biol. Sci. 2015, 11, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Pyron, R.A.; Wiens, J.J. A large-scale phylogeny of amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef] [PubMed]
- Jackway, R.J.; Pukala, T.L.; Donnellan, S.C.; Sherman, P.J.; Tyler, M.J.; Bowie, J.H. Skin peptide and cDNA profiling of Australian anurans: Genus and species identification and evolutionary trends. Peptides 2011, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Bininda-Emonds, O.R.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Vanhoye, D.; Bruston, F.; Nicolas, P.; Amiche, M. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 2003, 270, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Diederik, H.; Calvo, X.R.; Verhaert, P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Proteom. 2014, 4. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Rocha-Resende, C.; Silva, D.M.; Ianzer, D.; Martin-Eauclaire, M.F.; Bougis, P.E.; de Lima, M.E.; Santos, R.A.; Pimenta, A.M. Tityus serrulatus hypotensins: A new family of peptides from scorpion venom. Biochem. Biophys. Res. Commun. 2008, 371, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Li, B.; Chen, T.; Kwok, H.F. A review on bradykinin-related peptides isolated from amphibian skin secretion. Toxins 2015, 7, 951–970. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Xi, X.; Wang, L.; Gao, Y.; Ma, C.; Chen, H.; Zhou, M.; Chen, T.; Shaw, C. Baltikinin: A New Myotropic Tryptophyllin-3 Peptide Isolated from the Skin Secretion of the Purple-Sided Leaf Frog, Phyllomedusa baltea. Toxins 2016, 8, 213. https://doi.org/10.3390/toxins8070213
Shi D, Xi X, Wang L, Gao Y, Ma C, Chen H, Zhou M, Chen T, Shaw C. Baltikinin: A New Myotropic Tryptophyllin-3 Peptide Isolated from the Skin Secretion of the Purple-Sided Leaf Frog, Phyllomedusa baltea. Toxins. 2016; 8(7):213. https://doi.org/10.3390/toxins8070213
Chicago/Turabian StyleShi, Daning, Xinping Xi, Lei Wang, Yitian Gao, Chengbang Ma, Hang Chen, Mei Zhou, Tianbao Chen, and Chris Shaw. 2016. "Baltikinin: A New Myotropic Tryptophyllin-3 Peptide Isolated from the Skin Secretion of the Purple-Sided Leaf Frog, Phyllomedusa baltea" Toxins 8, no. 7: 213. https://doi.org/10.3390/toxins8070213





