The Saccharomyces boulardii CNCM I-745 Strain Shows Protective Effects against the B. anthracis LT Toxin
Abstract
:1. Introduction
2. Results
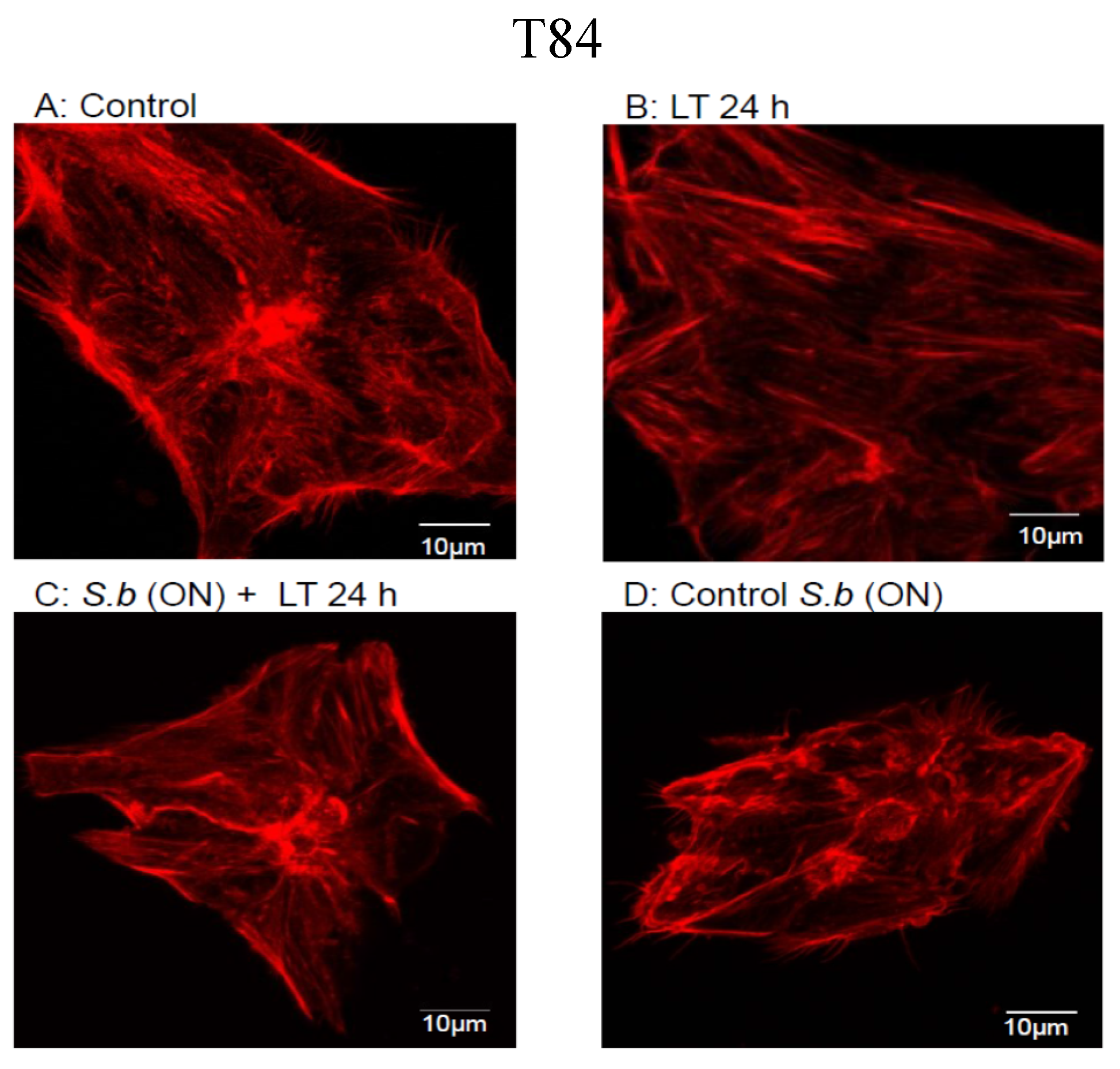
2.1. S. boulardii Protects against Cell Intoxication by LT

2.2. S. boulardii Prevents LT-Induced Loss in Permeability
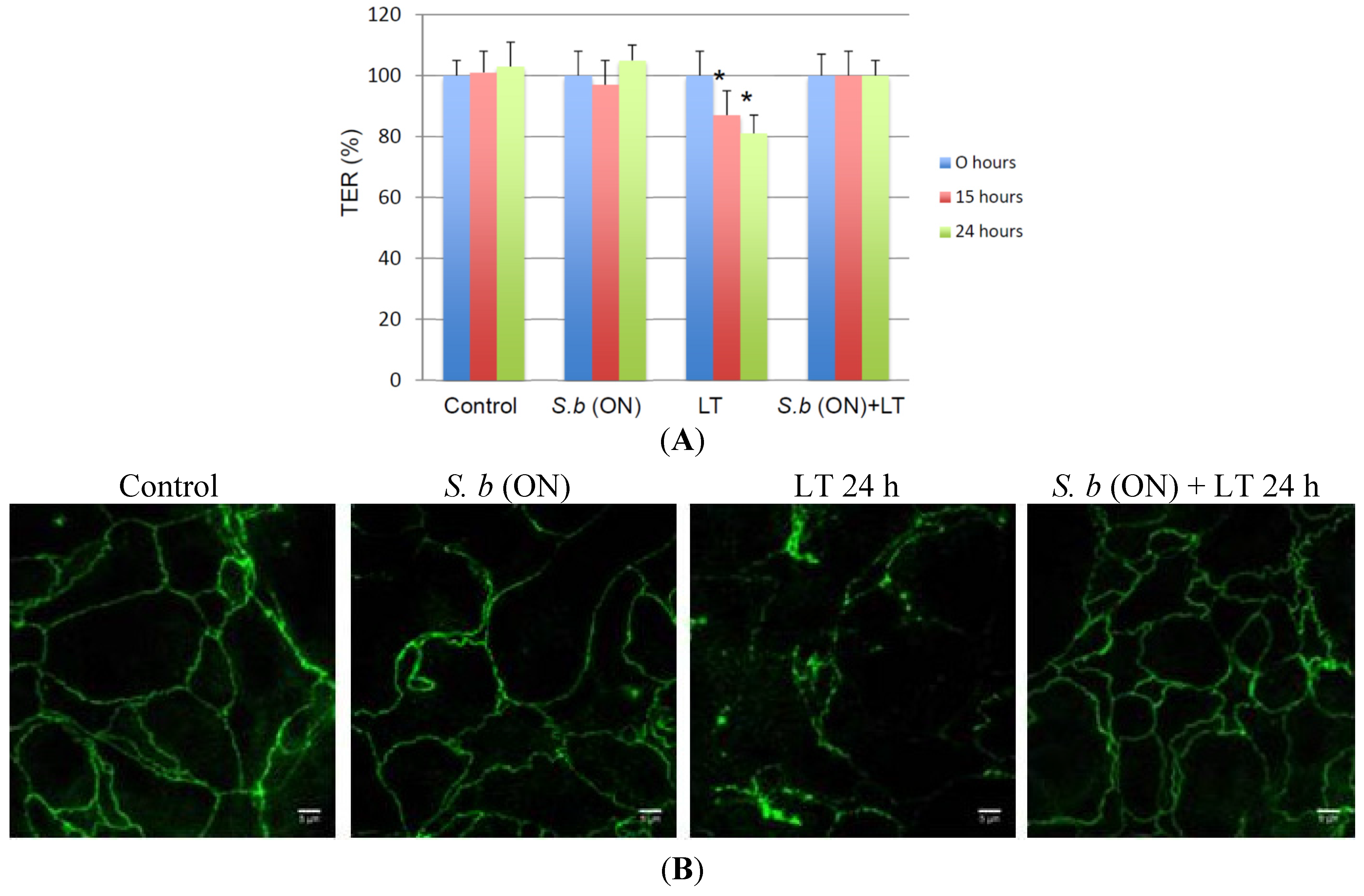
2.3. S. boulardii Induces Protective Effects on LT-Induced MEK-2 Cleavage
2.4. S. boulardii Interacts with PA and LF
3. Discussion
4. Material and Methods
4.1. Cell Lines and Growth Conditions
4.2. Microorganisms
4.3. Toxins
4.4. Trans-Epithelial Resistance Measurements
4.5. Interaction of Yeast with PA or LF
4.6. Western Blotting
4.7. Immunofluorescence Analyses
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mock, M.; Fouet, A. Anthrax. Annu. Rev. Microbiol. 2001, 55, 647–671. [Google Scholar] [CrossRef] [PubMed]
- Beatty, M.E.; Ashford, D.A.; Griffin, P.M.; Tauxe, R.V.; Sobel, J. Gastrointestinal anthrax: Review of the literature. Arch. Intern. Med. 2003, 163, 2527–2531. [Google Scholar] [CrossRef] [PubMed]
- Abramova, F.A.; Grinberg, L.M.; Yampolskaya, O.V.; Walker, D.H. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 1993, 90, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.A.; Hicks, C.W.; Cui, X.; Li, Y.; Eichacker, P.Q. Anthrax infection. Am. J. Respir. Crit. Care Med. 2011, 184, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Leppla, S.H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 2009, 30, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Young, J.A. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 2003, 19, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Trescos, Y.; Tournier, J.N. Cytoskeleton as an emerging target of anthrax toxins. Toxins 2012, 4, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Leppla, S.H. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166. [Google Scholar] [CrossRef] [PubMed]
- Duesbery, N.S.; Webb, C.P.; Leppla, S.H.; Gordon, V.M.; Klimpel, K.R.; Copeland, T.D.; Ahn, N.G.; Oskarsson, M.K.; Fukasawa, K.; Paull, K.D.; et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 1998, 280, 734–737. [Google Scholar] [PubMed]
- Chavarria-Smith, J.; Vance, R.E. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 2013, 9, e1003452. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.M.; Steele, A.D.; D’Agnillo, F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 2005, 166, 1871–1881. [Google Scholar] [CrossRef]
- Rolando, M.; Stefani, C.; Flatau, G.; Auberger, P.; Mettouchi, A.; Mhlanga, M.; Rapp, U.; Galmiche, A.; Lemichez, E. Transcriptome dysregulation by anthrax lethal toxin plays a key role in induction of human endothelial cell cytotoxicity. Cell. Microbiol. 2010, 12, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Stefani, C.; Doye, A.; Acosta, M.I.; Visvikis, O.; Yevick, H.G.; Buchrieser, C.; Mettouchi, A.; Bassereau, P.; Lemichez, E. Contractile actin cables induced by Bacillus anthracis lethal toxin depend on the histone acetylation machinery. Cytoskeleton 2015. [Google Scholar] [CrossRef]
- Trescos, Y.; Tessier, E.; Rougeaux, C.; Goossens, P.L.; Tournier, J.N. Micropatterned macrophage analysis reveals global cytoskeleton constraints induced by Bacillus anthracis edema toxin. Infect. Immun. 2015, 83, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap. Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; LaMont, J.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect. Immun. 1999, 67, 302–307. [Google Scholar] [CrossRef]
- Pothoulakis, C.; Kelly, C.P.; Joshi, M.A.; Gao, N.; O'Keane, C.J.; Castagliuolo, I.; Lamont, J.T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 1993, 104, 1108–1115. [Google Scholar] [PubMed]
- Buts, J.P.; Dekeyser, N.; Stilmant, C.; Delem, E.; Smets, F.; Sokal, E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr. Res. 2006, 60, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Brandao, R.L.; Castro, I.M.; Bambirra, E.A.; Amaral, S.C.; Fietto, L.G.; Tropia, M.J.; Neves, M.J.; Dos Santos, R.G.; Gomes, N.C.; Nicoli, J.R. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1998, 64, 564–568. [Google Scholar] [PubMed]
- Czerucka, D.; Rampal, P. Effect of Saccharomyces boulardii on cAMP- and Ca2+ -dependent Cl- secretion in T84 cells. Dig. Dis. Sci. 1999, 44, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Roux, I.; Rampal, P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology 1994, 106, 65–72. [Google Scholar] [PubMed]
- Chen, X.; Kokkotou, E.G.; Mustafa, N.; Bhaskar, K.R.; Sougioultzis, S.; O’Brien, M.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J. Biol. Chem. 2006, 281, 24449–24454. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Noack, D.; Wood, M.; Perego, M.; Knaus, U.G. Lung epithelial injury by B. anthracis lethal toxin is caused by MKK-dependent loss of cytoskeletal integrity. PLoS One 2009, 4, e4755. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Munro, P.; Stefani, C.; Auberger, P.; Flatau, G.; Lemichez, E. Injection of Staphylococcus aureus EDIN by the Bacillus anthracis protective antigen machinery induces vascular permeability. Infect. Immun. 2009, 77, 3596–3601. [Google Scholar] [PubMed]
- Huang, B.; Xie, T.; Rotstein, D.; Fang, H.; Frucht, D.M. Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax. Toxins 2015, 7, 3960–3976. [Google Scholar] [CrossRef] [PubMed]
- Beitzinger, C.; Bronnhuber, A.; Duscha, K.; Riedl, Z.; Huber-Lang, M.; Benz, R.; Hajós, G.; Barth, H. Designed azolopyridinium salts block protective antigen pores in vitro and protect cells from anthrax toxin. PLoS One 2013, 8, e66099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhang, Y.; Moayeri, M.; Liu, J.; Crown, D.; Fattah, R.J.; Wein, A.N.; Yu, Z.X.; Finkel, T.; Leppla, S.H. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature 2013, 501, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, H.; Xie, T.; Auth, R.D.; Patel, N.; Murray, P.R.; Snoy, P.J.; Frucht, D.M. Anthrax lethal toxin disrupts intestinal barrier function and causes systemic infections with enteric bacteria. PLoS One 2012, 7, e33585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guichard, A.; McGillivray, S.M.; Cruz-Moreno, B.; van Sorge, N.M.; Nizet, V.; Bier, E. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature 2010, 467, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Auth, R.D.; Frucht, D.M. The effects of anthrax lethal toxin on host barrier function. Toxins 2011, 3, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K. Furin: A mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 1997, 327, 625–635. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontier-Bres, R.; Rampal, P.; Peyron, J.-F.; Munro, P.; Lemichez, E.; Czerucka, D. The Saccharomyces boulardii CNCM I-745 Strain Shows Protective Effects against the B. anthracis LT Toxin. Toxins 2015, 7, 4455-4467. https://doi.org/10.3390/toxins7114455
Pontier-Bres R, Rampal P, Peyron J-F, Munro P, Lemichez E, Czerucka D. The Saccharomyces boulardii CNCM I-745 Strain Shows Protective Effects against the B. anthracis LT Toxin. Toxins. 2015; 7(11):4455-4467. https://doi.org/10.3390/toxins7114455
Chicago/Turabian StylePontier-Bres, Rodolphe, Patrick Rampal, Jean-François Peyron, Patrick Munro, Emmanuel Lemichez, and Dorota Czerucka. 2015. "The Saccharomyces boulardii CNCM I-745 Strain Shows Protective Effects against the B. anthracis LT Toxin" Toxins 7, no. 11: 4455-4467. https://doi.org/10.3390/toxins7114455




