Subchronic Toxicity Evaluation of Ethanol Extract of Picria fel-terrae Lour. Leaf in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Extraction and Characterization
2.2. Chemical Reagents and Tools
2.3. Animals
2.4. Subchronic Toxicity Study
- Na-CMC suspension 0.5% w/v.
- P. fel-terrae extract 125 mg/kg BW.
- P. fel-terrae extract 250 mg/kg BW.
- P. fel-terrae extract 500 mg/kg BW.
- P. fel-terrae extract 1000 mg/kg BW.
- Satellite control Na-CMC 0.5% w/v.
- Satellite Dose 1000 mg/kg BW.
2.5. Analysis of Hematological and Biochemistry
2.6. Macroscopic Assessment of the Organs
2.7. Microscopic Study of the Organs
2.8. Statistical Analysis
3. Results
3.1. Signs of Toxicity
3.2. Body Weight Observation
3.3. Mortality
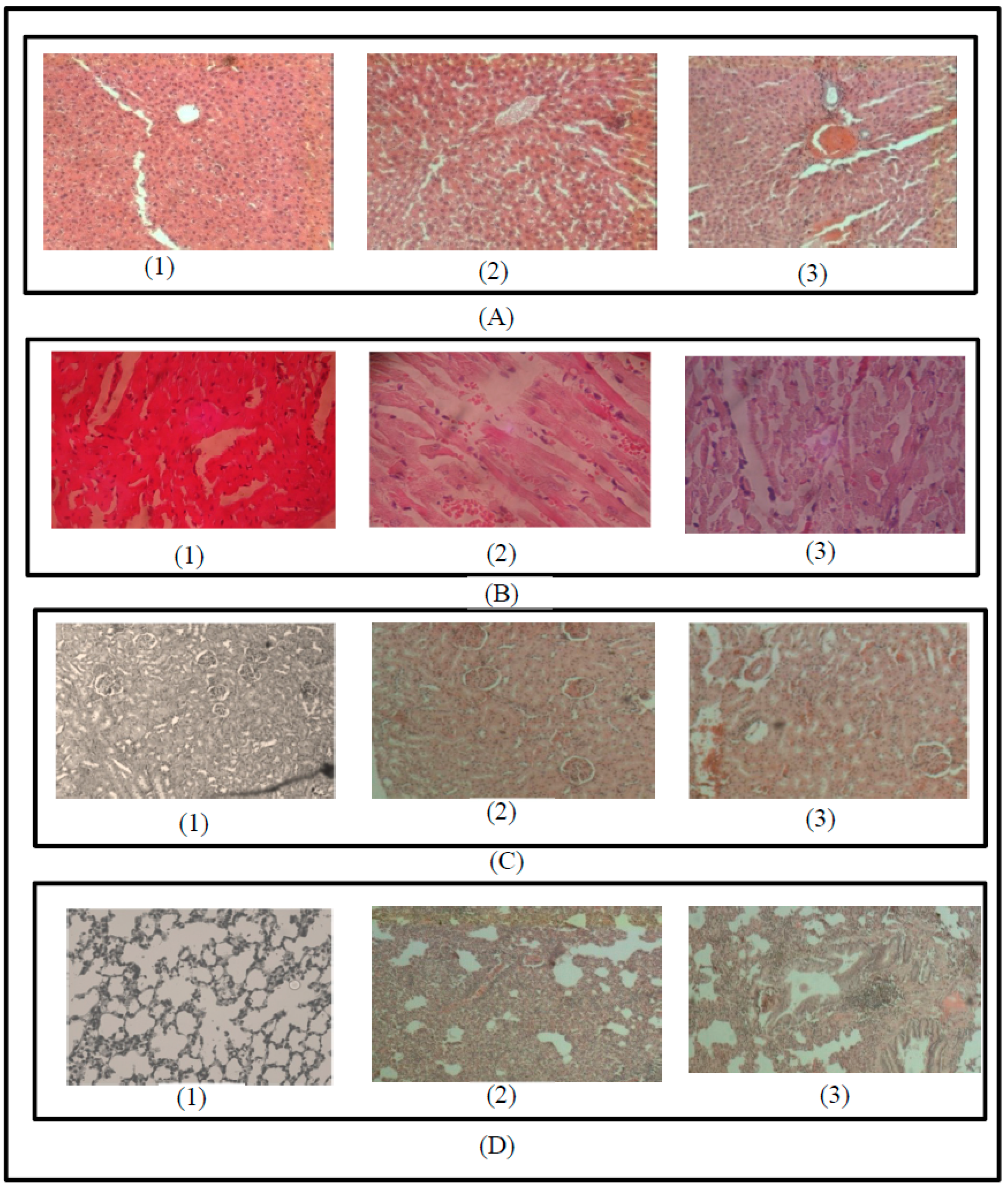
3.4. Macroscopic and Microscopic Examination
3.5. Biochemistry Parameters
3.6. Hematological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BW | body weight |
| OECD | Organisation for Economic Co-Operation and Development |
| BUN | blood urea nitrogen |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
References
- Schmelzer, G.H.; Horsten, S.F.A.J. Plant Resources of South-East Asia Leiden; Backhuys Publisher: Leiden, The Netherlands, 2001; pp. 426–428. [Google Scholar]
- Juwita, N.A.; Harahap, U.; Dalimunthe, A. Relaxation effect of ethanolic extract of Picria fel-terrae (Pugon tanoh) leaves on contraction of isolated rat’s ileum contracted by serotonin. J. Innov. Pharm. Biol. Sci. 2018, 5, 37–41. [Google Scholar]
- Kumarasingha, R.; Karpe, A.V.; Preston, S.; Yeo, T.C.; Lim, D.S.L.; Tu, C.L.; Luu, J.; Simpson, K.J.; Shaw, J.M.; Gasser, R.B.; et al. Metabolic profiling and in vitro assessment of anthelmintic fractions of Picria fel-terrae Lour. Int. J. Parasitol. Drugs 2016, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lindarto, D.; Syafril, S.; Zein, U.; Saragih, A. The Effect of dhawalsan-1 (Curanga fel-terrae [Lour.]) extract versus metformin on the metabolic and inflammatory characteristics of patients with newly diagnosed type 2 diabetes mellitus. Asian J. Pharm. Clin. Res. 2016, 9, 225–228. [Google Scholar]
- Dalimunthe, A.; Harahap, U.; Rosidah; Nasution, M.P. Evaluation of diuretic activity of Picria fel-terrae Lour leaves extracts. Asian J. Pharm. Clin. Res. 2015, 8, 204–205. [Google Scholar]
- Marianne; Chrestella, J.; Ginting, M.A.; Dalimunthe, A.; Nasution, R. Hepatoprotective activity combination of Curanga fel-terrae Lour leaves and Curcuma heyneana valeton and zijprhizome in rat induced by a combination of rifampin and isoniazid. Int. J. Pharm. 2017, 9, 23–28. [Google Scholar]
- Ryle, P.R.; Chakraborty, J.; Thomson, A.D. Biochemical mode of action of a hepatoprotective drug: Observations on (+)-catechin. Pharmacol. Biochem. Behav. 1983, 18, 473–478. [Google Scholar] [CrossRef]
- Jordan, S.A.; Cunningham, D.G.; Marles, R.J. Assessment of herbal medicinal products: Challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol. Appl. Pharmacol. 2010, 243, 198–216. [Google Scholar] [CrossRef] [PubMed]
- OECD. Organization for Economic Cooperation and Development Guidelines for the Testing of Chemicals TG 407; OECD: Paris, France, 2008; pp. 4–13. [Google Scholar]
- OECD. Repeated Dose 90-Day Oral Toxicity Study in Rodents TG 408; OECD: Paris, France, 2008. [Google Scholar]
- Kayarohanam, S.; Kavimani, S. Acute and sub-acute toxicity study of aqueous methanolic leaf and bark extract of Dolichandrone atrovirens. Int. J. Pharm. 2015, 7, 63–65. [Google Scholar]
- CRL (Charles River Laboratories) Technical Bulletin. Baseline Hematology and Clinical Chemistry Values for Charles River Wistar Rats (CRL:(WI)BR) as a Function of Sex and Age; Charles River Laboratories: Wilmington, DE, USA, 1998. [Google Scholar]
- Gupta, D.; Bhardwaj, S. Study of Acute, Subacute and Chronic Toxicity Test. Int. J. Adv. Res. Pharm. Biol. Sci. 2012, 1, 103–114. [Google Scholar]
- Hastuti, U.S. The Influence of Various Dosages of Citrinin on Hepatocyte Structure Mice (Mus Musculus) Damage to Three Lobulus Hepar Zone. J. Kedokteran Brawijaya 2006, 22, 121–124. [Google Scholar]
- Blachley, J.D.; Johnson, J.H.; Knochel, J.P. The harmful effects of ethanol on ion transport and cellular respiration. Am. J. Med Sci. 1985, 289, 22–26. [Google Scholar] [CrossRef] [PubMed]
- George, A.J. Legal Status and Toxicity of Saponins. Food Cosmet. Toxicol. 1965, 3, 85–89. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Procházkováa, D.; Boušová, I.; Wilhelmováa, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K.; Jarrar, B.M. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011, 10, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group | Gender | Weeks | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| I | Female | 155.10 ± 1.31 | 160.80 ± 2.43 | 166.14 ± 2.46 | 175.84 ± 2.52 |
| Male | 151.90 ± 4.62 | 167.86 ± 1.60 | 171.32 ± 2.10 | 178.56 ± 4.74 | |
| II | Female | 153.98 ± 4.88 | 163.54 ± 2.06 | 176.78 ± 3.89 | 176.78 ± 3.89 |
| Male | 151.44 ± 3.96 | 163.88 ± 2.67 | 173.14 ± 5.07 | 173.14 ± 5.07 | |
| III | Female | 154.34 ± 4.98 | 166.72 ± 2.32 | 170.08 ± 2.64 | 177.52 ± 3.02 |
| Male | 155.86 ± 4.58 | 162.14 ± 3.38 | 165.02 ± 3.22 | 173.70 ± 2.81 | |
| IV | Female | 152.04 ± 4.60 | 163.20 ± 3.39 | 167.88 ± 3.62 | 178.56 ± 1.74 |
| Male | 149.54 ± 4.17 | 162.66 ± 1.87 | 163.70 ± 2.89 | 175.92 ± 4.16 | |
| V | Female | 150.94 ± 3.50 | 164.34 ± 1.31 | 169.48 ± 3.30 | 176.68 ± 4.16 |
| Male | 152.62 ± 2.96 | 164.62 ± 3.33 | 168.10 ± 2.23 | 177.26 ± 1.53 | |
| VI | Female | 157.72 ± 4.23 | 162.14 ± 4.43 | 172,98 ± 2.56 | 181.50 ± 2.06 |
| Male | 152.50 ± 3.47 | 159.56 ± 5.38 | 174,94 ± 2.58 | 183.00 ± 3.11 | |
| VII | Female | 155.02 ± 0.92 | 157.82 ± 1.19 | 158,86 ± 1.51 | 168.55 ± 2.53 |
| Male | 154.32 ± 2.19 | 158.64 ± 6.77 | 161,22 ± 5.14 | 174.12 ± 4.46 | |
| Group | Gender | Mean of Relative Organ Index (100 g) ± SD | ||||
|---|---|---|---|---|---|---|
| Liver | Spleen | Kidney | Lung | Heart | ||
| I | Female | 2.86 ± 0.15 | 0.24 ± 0.03 | 0.37 ± 0.04 | 0.37 ± 0.04 | 0.37 ± 0.01 |
| Male | 2.63 ± 0.32 | 0.22 ± 0.05 | 0.37 ± 0.03 | 0.37 ± 0.03 | 0.35 ± 0.01 | |
| II | Female | 4.44 ± 0.30 | 0.32 ± 0.01 | 0.37 ± 0.04 | 0.37 ± 0.04 | 0.37 ± 0.01 |
| Male | 3.58 ± 0.56 | 0.25 ± 0.01 | 0.38 ± 0.04 | 0.38 ± 0.04 | 0.35 ± 0.01 | |
| III | Female | 4.30 ± 0.53 | 0.35 ± 0.02 | 0.37 ± 0.03 | 0.37 ± 0.03 | 0.34 ± 0.02 |
| Male | 4.24 ± 0.42 | 0.26 ± 0.07 | 0.37 ± 0.02 | 0.37 ± 0.02 | 0.35 ± 0.01 | |
| IV | Female | 4.30 ± 0.53 * | 0.33 ± 0.02 | 0.37 ± 0.02 * | 0.37 ± 0.02 * | 0.34 ± 0.03 |
| Male | 4.24 ± 0.42 * | 0.28 ± 0.03 | 0.37 ± 0.03 * | 0.37 ± 0.03 * | 0.33 ± 0.01 | |
| V | Female | 4.03 ± 0.05 * | 0.29 ± 0.08 | 0.40 ± 0.05 * | 0.40 ± 0.05 * | 0.37 ± 0.00 |
| Male | 5.68 ± 1.66 * | 0.29 ± 0.09 | 0.41 ± 0.04 * | 0.41 ± 0.04 * | 0.36 ± 0.01 | |
| VI | Female | 3.97 ± 0.56 | 0.30 ± 0.04 | 0.39 ± 0.04 | 0.39 ± 0.04 | 0.31 ± 0.01 |
| Male | 3.92 ± 0.51 | 0.28 ± 0.03 | 0.39 ± 0.03 | 0.34 ± 0.03 | 0.33 ± 0.02 | |
| VII | Female | 4.01 ± 0.42 | 0.34 ± 0.03 | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.34 ± 0.02 |
| Male | 3.86 ± 0.71 | 0.26 ± 0.03 | 0.42 ± 0.02 | 0.42 ± 0.02 | 0.36 ± 0.02 | |
| Group | Gender | ALT | AST | Creatinin | BUN |
|---|---|---|---|---|---|
| I | Female | 58.20 ± 8.75 | 211.20 ± 4.6 | 0.58 ± 0.19 | 48.42 ± 6.045 |
| Male | 50.40 ± 6.65 | 207.80 ± 4.5 | 0.68 ± 0.32 | 50.66 ± 3.43 | |
| II | Femae | 58.60 ± 6.95 | 215.60 ± 2.0 | 0.56 ± 0.12 | 48.20 ± 8.53 |
| Male | 50.80 ± 6.49 | 211.00 ± 2.5 | 0.65 ± 0.12 | 49.82 ± 9.63 | |
| III | Femae | 63.80 ± 9.36 | 220.80 ± 3.3 | 0.53 ± 0.08 | 48.60 ± 9.91 |
| Male | 50.80 ± 4.14 | 216.80 ± 2.8 | 0.63 ± 0.14 | 64.80 ± 11.67 | |
| IV | Femae | 70.60 ± 7.82 * | 232.40 ± 7.7 * | 0.41 ± 0.15 * | 49.80 ± 13.55 * |
| Male | 54.25 ± 4.78 * | 216.80 ± 2.28 * | 0.65 ± 0.26 * | 42.50 ± 7.59 * | |
| V | Female | 78.40 ± 8.20 * | 246.80 ± 9.57 * | 0.60 ± 0.12 * | 55.20 ± 11.88 * |
| Male | 71.20 ± 10.20 * | 224.75 ± 2.98 * | 0.60 ± 0.20 * | 45.20 ± 9.52 * | |
| VI | Female | 56.00 ± 5.24 | 209.40 ± 4.82 | 0.42 ± 0.24 | 52.40 ± 10.14 |
| Male | 46.60 ± 7.33 | 207.20 ± 3.83 | 0.63 ± 0.12 | 59.60 ± 7.13 | |
| VII | Female | 62.60 ± 5.03 | 216.50 ± 3.69 | 0.61 ± 0.10 | 52.75 ± 0.96 |
| Male | 56.25 ± 5.79 | 217.25 ± 3.30 | 0.66 ± 0.13 | 49.50 ± 3.11 |
| Group | Gender | WBC | RBC | Platelet | Hemoglobin | Hematocrit | MCHC | MCV | MCH | Eosinofil | Monosit | Basofil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Female | 5.98 ± 0.99 | 7.93 ± 0.76 | 785.60 ± 117.02 | 15.72 ± 1.06 | 44.10 ± 3.33 | 34.78±1.58 | 54.04 ± 2.03 | 18.64 ± 0.88 | 3.22 ± 0.61 | 1.80 ± 0.84 | 0.36 ± 0.13 |
| Male | 6.84 ± 1.23 | 8.49 ± 1.23 | 895.80 ± 140.94 | 15.34 ± 1.25 | 47.08 ± 5.41 | 32.86 ± 0.99 | 54.88 ± 0.79 | 17.46 ± 0.68 | 3.16 ± 0.63 | 1.66 ± 0.63 | 0.39 ± 0.09 | |
| II | Female | 6.75 ± 1.23 | 7.94 ± 0.73 | 859.20 ± 189.49 | 15.48 ± 1.18 | 43.86 ± 3.99 | 35.54 ± 2.14 | 55.82 ± 1.93 | 18.78 ± 0.74 | 3.24 ± 0.71 | 2.16 ± 0.54 | 0.37 ± 0.10 |
| Male | 7.73 ± 1.62 | 8.95 ± 0.84 | 920.20 ± 175.93 | 15.16 ± 0.99 | 43.54 ± 4.91 | 33.58 ± 2.54 | 53.96 ± 3.34 | 17.84 ± 0.83 | 2.94 ± 0.58 | 1.82 ± 0.86 | 0.38 ± 0.09 | |
| III | Female | 7.40 ± 1.05 | 7.49 ± 0.43 | 847.40 ± 161.39 | 15.22 ± 0.91 | 45.22 ± 4.01 | 35.48 ± 1.64 | 57.14 ± 1.36 | 18.32 ± 0.70 | 3.22 ± 0.86 | 1.90 ± 0.89 | 0.35 ± 0.08 |
| Male | 8.24 ± 0.95 | 8.29 ± 1.04 | 919.40 ± 208.39 | 15.42 ± 1.26 | 46.34 ± 6.15 | 34.66 ± 2.52 | 54.62 ± 2.92 | 18.20 ± 0.82 | 3.03 ± 0.22 | 2.10 ± 0.64 | 0.37 ± 0.11 | |
| IV | Female | 7.83 ± 0.83 | 7.49 ± 0.81 | 898.40 ± 167.42 | 15.18 ± 1.12 | 45.18 ± 3.47 | 35.48 ± 1.64 | 57.50 ± 0.78 | 18.54 ± 0.50 | 3.22 ± 0.86 | 1.80 ± 0.67 | 0.36 ± 0.09 |
| Male | 8.42 ± 0.64 | 8.02 ± 1.17 | 886.25 ± 125.31 | 14.15 ± 1.15 | 48.53 ± 5.74 | 34.66 ± 2.52 | 55.30 ± 1.92 | 17.93 ± 0.83 | 3.03 ± 0.22 | 2.40 ± 0.76 | 0.38 ± 0.12 | |
| V | Female | 9.67 ± 1.16 | 6.83 ± 0.65 | 809.00 ± 207.26 | 15.18 ± 1.07 | 43.36 ± 3.09 | 35.42 ± 1.60 | 56.50 ± 0.93 | 18.64 ± 1.10 | 3.28 ± 0.63 | 2.40 ± 0.82 | 0.37 ± 0.10 |
| Male | 9.83 ± 0.79 | 6.99 ± 1.34 | 905.00 ± 203.02 | 14.62 ± 1.41 | 47.42 ± 6.37 | 34.86 ± 2.82 | 53.84 ± 3.07 | 17.42 ± 1.12 | 3.18 ± 0.58 | 2.50 ± 0.46 | 0.43 ± 0.11 | |
| VI | Female | 6.08 ± 0.77 | 7.96 ± 0.72 | 895.00 ± 88.66 | 15.78 ± 0.69 | 44.16 ± 3.31 | 34.52 ± 0.68 | 53.90 ± 1.77 | 18.70 ± 0.57 | 3.20 ± 0.60 | 1.98 ± 0.72 | 0.36 ± 0.07 |
| Male | 6.99 ± 1.43 | 8.42 ± 1.26 | 899.60 ± 85.93 | 5.22 ± 1.18 | 46.52 ± 3.78 | 33.36 ± 0.83 | 54.66 ± 0.48 | 17.40 ± 0.53 | 3.06 ± 0.62 | 1.70 ± 0.47 | 0.39 ± 0.03 | |
| VII | Female | 9.62 ± 0.62 | 6.88 ± 0.89 | 844.00 ± 195.88 | 15.70 ± 0.95 | 46.52 ± 3.78 | 35.95 ± 1.20 | 56.30 ± 1.29 | 18.50 ± 0.59 | 3.06 ± 0.62 | 2.53 ± 0.47 | 0.36 ± 0.06 |
| Male | 9.50 ± 0.71 | 7.01 ± 0.73 | 1003.75 ± 109.28 | 15.35 ± 0.64 | 51.65 ± 2.74 | 34.08 ± 2.31 | 52.99 ± 0.29 | 17.23 ± 0.64 | 3.18 ± 0.58 | 2.38 ± 0.53 | 0.43 ± 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harahap, U.; Yuandani; Marianne; Agustya, H.M.; Azizah, D.U.; Alfiah, S.W. Subchronic Toxicity Evaluation of Ethanol Extract of Picria fel-terrae Lour. Leaf in Wistar Rats. Sci. Pharm. 2018, 86, 34. https://doi.org/10.3390/scipharm86030034
Harahap U, Yuandani, Marianne, Agustya HM, Azizah DU, Alfiah SW. Subchronic Toxicity Evaluation of Ethanol Extract of Picria fel-terrae Lour. Leaf in Wistar Rats. Scientia Pharmaceutica. 2018; 86(3):34. https://doi.org/10.3390/scipharm86030034
Chicago/Turabian StyleHarahap, Urip, Yuandani, Marianne, Hafiza Mitha Agustya, Dira Ummul Azizah, and Syari Widia Alfiah. 2018. "Subchronic Toxicity Evaluation of Ethanol Extract of Picria fel-terrae Lour. Leaf in Wistar Rats" Scientia Pharmaceutica 86, no. 3: 34. https://doi.org/10.3390/scipharm86030034