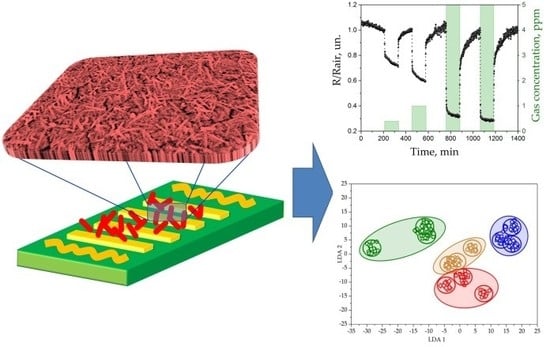
The Multisensor Array Based on Grown-On-Chip Zinc Oxide Nanorod Network for Selective Discrimination of Alcohol Vapors at Sub-ppm Range
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Analytical Characterization of ZnO NR Network
3.2. The Electrical Characterization of ZnO NR Network Under Air Conditions
3.3. The Gas-Sensing Characterization of ZnO NR Network Upon Exposure to Alcohol Vapors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, A.; Edwards, P. Validate personal air-pollution sensors. Nature 2016, 535, 29–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupriyanov, L.Y. (Ed.) Semiconductor Sensors in Physico-Chemical Studies; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Korotcenkov, G.; Sysoev, V. Conductometric metal oxide gas sensors: Principles of operation and approaches to fabrication. In Chemical Sensors: Comprehensive Sensor Technologies; Korotcenkov, G., Ed.; Volume 4: Solid State Devices; Momentum Press: New York, NY, USA, 2011; pp. 53–186. [Google Scholar]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatahi, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Taguchi, N. Gas Detecting Device. U.S. Patent 3,695,848, 3 October 1972. [Google Scholar]
- Wolkenstein, T. Electronic Processes on Semiconductor Surfaces during Chemisorption; Consultants Bureau: New York, NY, USA, 1991; p. 460. [Google Scholar]
- Kissine, V.V.; Sysoev, V.V.; Voroshilov, S.A. Individual and collective effects of oxygen and ethanol on the conductance of SnO2 thin films. Appl. Phys. Lett. 2000, 76, 2391–2393. [Google Scholar] [CrossRef]
- Rothschild, A.; Komem, Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar] [CrossRef]
- Ahmad, R.; Majhi, S.M.; Zhang, X.; Swager, T.M.; Salama, K.N. Recent progress and perspectives of gas sensors based on vertically oriented ZnO nanomaterials. Adv. Colloid Interface Sci. 2019, 270, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Kang, B.S.; Wang, H.T.; Tien, L.C.; Ren, F.; Gila, B.P.; Norton, D.P.; Abernathy, C.R.; Lin, J.; Pearton, S.J. Wide bandgap semiconductor nanorod and thin film gas sensors. Sensors 2006, 6, 643–666. [Google Scholar] [CrossRef]
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 2004, 34, 151–180. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Oezguer, U.; Hofstetter, D.; Morkoc, H. ZnO devices and applications: A review of current status and future prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Udom, I.; Ram, M.K.; Stefanakos, E.K.; Hepp, A.F.; Goswami, D.Y. One dimensional-ZnO nanostructures: Synthesis, properties and environmental applications. Mater. Sci. Semicond. Process. 2013, 16, 2070–2083. [Google Scholar] [CrossRef]
- Pascariu, P.; Homocianu, M. ZnO-based ceramic nanofibers: Preparation, properties and applications. Ceram. Int. 2019, 15, 11158–11173. [Google Scholar] [CrossRef]
- Baratto, C. Growth and properties of ZnO nanorods by RF sputtering for detection of toxic gases. RSC Adv. 2018, 8, 32038. [Google Scholar] [CrossRef]
- Wang, H.T.; Kang, B.S.; Ren, F.; Tien, L.C.; Sadik, P.W.; Norton, D.P.; Pearton, S.J.; Lin, J. Hydrogen-selective sensing at room temperature with ZnO nanorods. Appl. Phys. Lett. 2005, 86, 243503. [Google Scholar] [CrossRef]
- Kuo, C.G.; Chen, J.H.; Chao, Y.C.; Chen, P.L. Fabrication of a P3HT-ZnO nanowires gas sensor detecting ammonia gas. Sensors 2018, 18, 37. [Google Scholar] [CrossRef]
- Yan, D.; Hu, M.; Li, S.; Liang, J.; Wu, Y.; Ma, S. Electrochemical deposition of ZnO nanostructures onto porous silicon and their enhanced gas sensing to NO2 at room temperature. Electrochim. Acta 2014, 115, 297–305. [Google Scholar] [CrossRef]
- Bai, S.; Sun, C.; Guo, T.; Luo, R.; Lin, Y.; Chen, A.; Sun, L.; Zhang, J. Low temperature electrochemical deposition of nanoporous ZnO thin films as novel NO2 sensors. Electrochim. Acta 2013, 90, 530–534. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Room temperature ethanol sensing properties of ZnO nanorods prepared using an electrospinning technique. J. Mater. Chem. C 2017, 5, 10869. [Google Scholar] [CrossRef]
- Choudharya, S.; Annapoornia, S.; Malik, R. Evolution and growth mechanism of hexagonal ZnO nanorods and their LPG sensing response at low operating temperature. Sens. Actuators A Phys. 2019, 293, 207–214. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.H.; Bak, S.Y.; Kim, Y.; Woo, K.; Lee, S.; Lim, Y.; Yi, M. Improved sensitivity of α-Fe2O3 nanoparticle-decorated ZnO nanowire gas sensor for CO. Sensors 2019, 19, 1903. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015, 63, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Chen, X.; Deng, Z.X.; Li, Y.D. CTAB-assisted hydrothermal orientation growth of ZnO nanorods. Mater. Chem. Phys. 2002, 78, 99–104. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.D.; Tsai, J.K.; Tang, J.Y.; Meen, T.H.; Wu, T.C. Zinc-oxide nanorod array fabricated by high temperature hydrothermal method applied to gas sensor. Microsyst. Technol. 2018, 24, 3957–3963. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Xu, L.; Peng, S. Hydrothermal synthesis of various hierarchical ZnO nanostructures and their methane sensing properties. Sensors 2013, 13, 6171–6182. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.K.; Porte, Y.; Ko, K.Y.; Kim, H.; Myoung, J.M. High-performance flexible ZnO nanorod UV/gas dual sensors using Ag nanoparticle templates. ACS Appl. Mater. Interfaces 2018, 10, 31505–31514. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, S.B.; Patil, V.L.; Vanalakar, S.A.; Patil, P.S.; Deshmukh, H.P. Preparation, characterization of 1D ZnO nanorods and their gas sensing properties. Ceram. Int. 2018, 44, 3333–3340. [Google Scholar] [CrossRef]
- Singh, P.; Hu, L.L.; Zan, H.W.; Tseng, T.Y. Highly sensitive nitric oxide gas sensor based on ZnO-nanorods vertical resistor operated at room temperature. Nanotechnology 2019, 30, 095501. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Lin, C.; Zhang, J. AuNPs hybrid black ZnO nanorods made by a sol-gel method for highly sensitive humidity sensing. Sensors 2018, 18, 218. [Google Scholar] [CrossRef]
- Yadav, A.B.; Parvathi, P.V.; Thabassum, S.R. Effect of precursor chemistry on the structural and sensing properties of hydrothermally grown nanorods. Appl. Phys. A 2019, 125, 446. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Huang, H.; Lee, Y.C.; Tan, O.K.; Vayssieres, L. Hydrothermally grown oriented ZnO nanorod arrays for gas sensing applications. Nanotechnology 2006, 17, 4995–4998. [Google Scholar] [CrossRef]
- Bie, L.J.; Yan, X.N.; Yin, J.; Duan, Y.Q.; Yuan, Z.H. Nanopillar ZnO gas sensor for hydrogen and ethanol. Sens. Actuators B 2007, 126, 604–608. [Google Scholar] [CrossRef]
- Quy, C.T.; Hung, C.M.; Duy, N.V.; Hoa, N.D.; Jiao, M.; Nguyen, H. Ethanol-sensing characteristics of nanostructured ZnO: Nanorods, nanowires, and porous nanoparticles. J. Electron. Mater. 2017, 46, 3406–3411. [Google Scholar] [CrossRef]
- Liu, J.; Yi, F. Fabrication and properties of ZnO nanorods on silicon nanopillar surface for gas sensor application. J. Mater. Sci. Mater. Electron. 2019, 30, 11404–11411. [Google Scholar] [CrossRef]
- Ridha, N.J.; Alosfur, F.K.M.; Jumali, M.H.H.; Radiman, S. Dimensional effect of ZnO nanorods on gas-sensing performance. J. Phys. D Appl. Phys. 2018, 51, 435101. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W.; Li, Y. New insight into gas sensing property of ZnO nanorods and nanosheets. Mater. Lett. 2018, 228, 331–333. [Google Scholar] [CrossRef]
- Jiaqiang, X.; Yuping, C.; Daoyong, C.; Jianian, S. Hydrothermal synthesis and gas sensing characters of ZnO nanorods. Sens. Actuators B Chem. 2006, 113, 526–531. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Ali, R.N.; Diao, K.; Naz, H.; Cui, X.; Xiang, B. Synthesis, characterization, and applications of zinc oxide nanoparticles and nanorods in acetone gas detection. Mater. Res. Express 2017, 4, 095015. [Google Scholar] [CrossRef] [Green Version]
- Hadiyan, M.; Salehi, A.; Koohi-Saadi, A. Sub-ppm acetone gas sensing properties of free-standing ZnO nanorods. J. Electroceramics 2019, 42, 147–155. [Google Scholar] [CrossRef]
- Gao, R.; Cheng, X.; Gao, S.; Zhang, X.; Xu, Y.; Zhao, H.; Huo, L. Highly selective detection of saturated vapors of abused drugs by ZnO nanorod bundles gas sensor. Appl. Surf. Sci. 2019, 485, 266–273. [Google Scholar] [CrossRef]
- Zhou, F.; Jing, W.; Liu, P.; Han, D.; Jiang, Z.; Wei, Z. Doping Ag in ZnO nanorods to improve the performance of related enzymatic glucose sensors. Sensors 2017, 17, 2214. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 211–221. [Google Scholar] [CrossRef]
- Goschnick, J. An electronic nose for intelligent consumer products based on a gas analytical gradient microarray. Microelectron. Eng. 2001, 57–58, 693–704. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Kiselev, I.; Frietsch, M.; Goschnick, J. Temperature gradient effect on gas discrimination power of a metal-oxide thin-film sensor microarray. Sensors 2004, 4, 37–46. [Google Scholar] [CrossRef]
- Bobkov, A.A.; Maximov, A.I.; Moshnikov, V.A.; Somov, P.A.; Terukov, E.I. Zinc-oxide-based nanostructured materials for heterostructure solar cells. Semiconductors 2015, 49, 1357–1360. [Google Scholar] [CrossRef]
- Lashkova, N.A.; Maximov, A.I.; Ryabko, A.A.; Bobkov, A.A.; Moshnikov, V.A.; Terukov, E.I. Synthesis of ZnO-based nanostructures for heterostructure photovoltaic cells. Semiconductors 2016, 50, 1254–1260. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Fedorov, F.; Podgainov, D.; Varezhnikov, A.; Lashkov, A.; Gorshenkov, M.; Burmistrov, I.; Sommer, M.; Sysoev, V. The potentiodynamic bottom-up growth of the tin oxide nanostructured layer for gas-analytical multisensor array chips. Sensors 2017, 17, 1908. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, F.S.; Varezhnikov, A.S.; Kiselev, I.; Kolesnichenko, V.V.; Burmistrov, I.N.; Sommer, M.; Fuchs, D.; Kübel, C.; Gorokhovsky, A.V.; Sysoev, V.V. Potassium polytitanate gas-sensor study by impedance spectroscopy. Anal. Chim. Acta 2015, 897, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, V.V.; Strelcov, E.; Sommer, M.; Bruns, M.; Kiselev, I.; Habicht, W.; Kar, S.; Gregoratti, L.; Kiskinova, M.; Kolmakov, A. Single-nanobelt electronic nose: Engineering and tests of the simplest analytical element. ACS Nano 2010, 4, 4487–4494. [Google Scholar] [CrossRef] [PubMed]
- Washenfelder, R.A.; Roehl, C.M.; McKinney, K.A.; Julian, R.R.; Wennberg, P.O. A compact, lightweight gas standards generator for permeation tubes. Rev. Sci. Instrum. 2003, 74, 3151–3154. [Google Scholar] [CrossRef] [Green Version]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-order chemical sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef]
- Fedorov, F.; Vasilkov, M.; Lashkov, A.; Varezhnikov, A.; Fuchs, D.; Kübel, C.h.; Bruns, M.; Sommer, M.; Sysoev, V. Toward new gas-analytical multisensor chips based on titanium oxide nanotube array. Sci. Rep. 2017, 7, 9732. [Google Scholar] [CrossRef] [Green Version]
- Sysoev, V.V.; Strelcov, E.; Kar, S.; Kolmakov, A. The electrical characterization of a multi-electrode odor detection sensor array based on the single SnO2 nanowire. Thin Solid Film. 2011, 520, 898–903. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on a thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, C.L.; Xiao, G. Reduced-temperature ethanol sensing characteristics of flower-like ZnO nanorods synthesized by a sonochemical method. Nanotechnology 2006, 17, 4537–4541. [Google Scholar] [CrossRef]
- Althagafi, T.M.; Al Baroot, A.F.; Grell, M. A new precursor route to semiconducting zinc oxide. IEEE Electron Device Lett. 2016, 37, 1299–1302. [Google Scholar] [CrossRef]
- Barreca, D.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E. ZnO nanoplatelets obtained by chemical vapor deposition, studied by XPS. Surf. Sci. Spectra 2007, 14, 19. [Google Scholar] [CrossRef]
- Beekmans, N.M.; Heyne, L. Correlation between impedance, microstructure and composition of calcia-stabilized zirconia. Electrochim. Acta 1976, 21, 303–310. [Google Scholar] [CrossRef]
- Van Dijk, T.; Burggraaf, A.J. Grain boundary effects on ionic conductivity in ceramic GdxZr1–XO2–(x/2) solid solutions. Phys. Status Solidi 1981, 63, 229–240. [Google Scholar] [CrossRef]
- Singh, R.C.; Singh, O.; Singh, M.P.; Chandic, P.S. Synthesis of zinc oxide nanorods and nanoparticles by chemical route and their comparative study as ethanol sensors. Sens. Actuators B Chem. 2008, 135, 352–357. [Google Scholar] [CrossRef]
- Karpova, S.S.; Moshnikov, V.A.; Mjakin, S.V.; Kolovangina, E.S. Surface functional composition and sensor properties of ZnO, Fe2O3, and ZnFe2O4. Semiconductors 2013, 47, 392–395. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Goschnick, J.; Schneider, T.; Strelcov, E.; Kolmakov, A. A gradient microarray electronic nose based on percolating SnO2 nanowire sensing elements. Nano Lett. 2007, 7, 3182–3188. [Google Scholar] [CrossRef]
- Kiselev, I.; Sysoev, V.; Kaikov, I.; Koronczi, I.; Tegin, R.A.A.; Smanalieva, J.; Sommer, M.; Ilicali, C.; Hauptmannl, M. On temporal stability of analyte recognition with E-nose based on metal oxide sensor array in practical applications. Sensors 2018, 18, 550. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobkov, A.; Varezhnikov, A.; Plugin, I.; Fedorov, F.S.; Trouillet, V.; Geckle, U.; Sommer, M.; Goffman, V.; Moshnikov, V.; Sysoev, V. The Multisensor Array Based on Grown-On-Chip Zinc Oxide Nanorod Network for Selective Discrimination of Alcohol Vapors at Sub-ppm Range. Sensors 2019, 19, 4265. https://doi.org/10.3390/s19194265
Bobkov A, Varezhnikov A, Plugin I, Fedorov FS, Trouillet V, Geckle U, Sommer M, Goffman V, Moshnikov V, Sysoev V. The Multisensor Array Based on Grown-On-Chip Zinc Oxide Nanorod Network for Selective Discrimination of Alcohol Vapors at Sub-ppm Range. Sensors. 2019; 19(19):4265. https://doi.org/10.3390/s19194265
Chicago/Turabian StyleBobkov, Anton, Alexey Varezhnikov, Ilya Plugin, Fedor S. Fedorov, Vanessa Trouillet, Udo Geckle, Martin Sommer, Vladimir Goffman, Vyacheslav Moshnikov, and Victor Sysoev. 2019. "The Multisensor Array Based on Grown-On-Chip Zinc Oxide Nanorod Network for Selective Discrimination of Alcohol Vapors at Sub-ppm Range" Sensors 19, no. 19: 4265. https://doi.org/10.3390/s19194265