Fast Phenomics in Vineyards: Development of GRover, the Grapevine Rover, and LiDAR for Assessing Grapevine Traits in the Field
Abstract
:1. Introduction
2. Materials and Methods
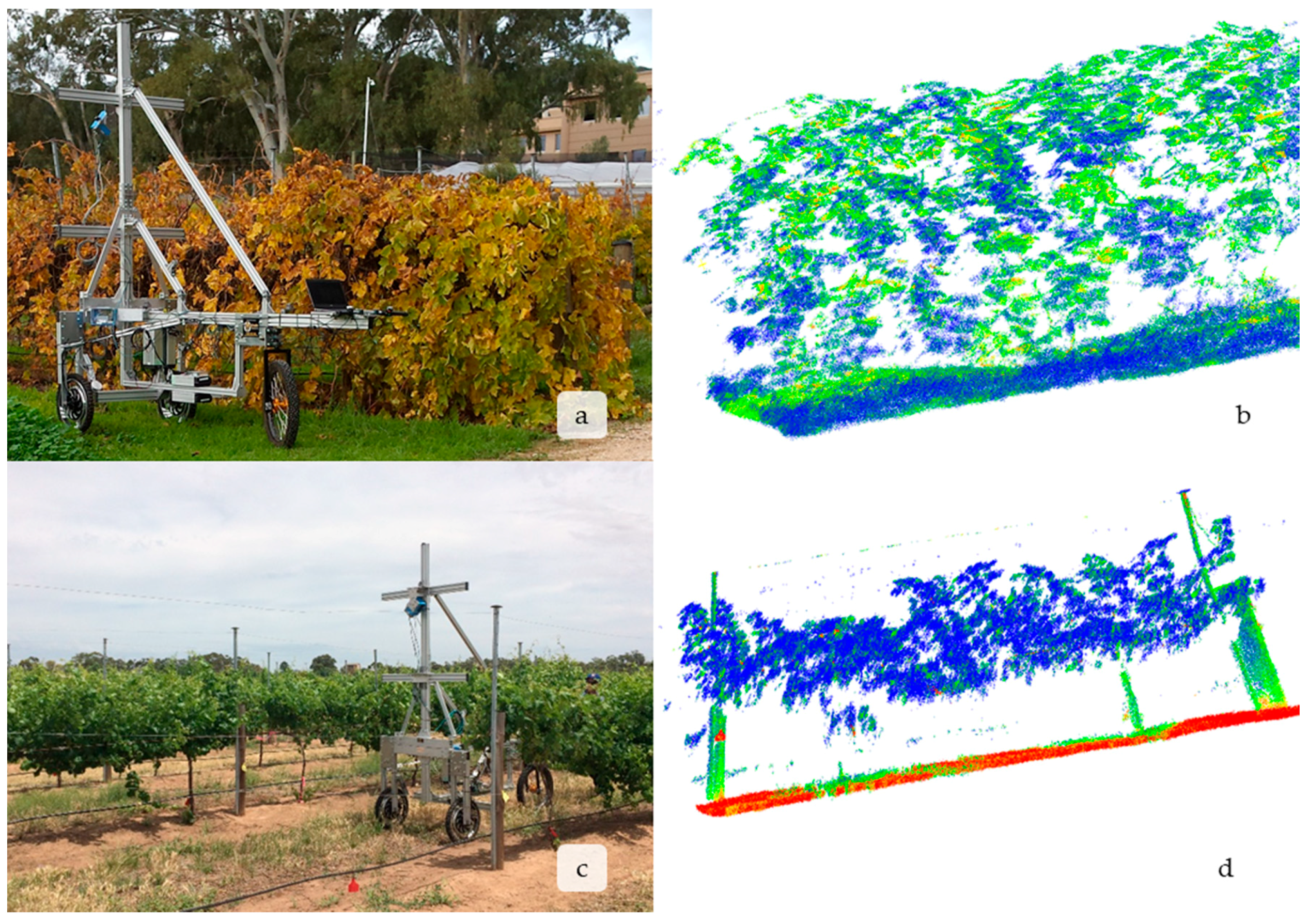
2.1. Description of the GRover’s Platform
2.2. LiDAR Sensor Specifications
2.3. Scans and Voxelization
2.4. Data Processing
2.5. Vineyards
- -
- Alverstoke teaching vineyard, University of Adelaide, Waite Campus, Adelaide, South Australia. Variety Shiraz, trained to a single cordon and spur or minimally pruned. A single foliage wire was used to vertically shoot position the vines during the growing season. This has a mildly sloping terrain with a winter mid-row cover crop;
- -
- South Australian Research and Development Institute (SARDI) research vineyard at Nuriootpa, South Australia. Variety Shiraz, trained to a single cordon and spur pruned. Vines were allowed to ”sprawl” during the growing season. Flat terrain. One planting of the Shiraz variety at this location was used for comparison of LiDAR scans with cordon and trunk volume measurements and another used for comparison with pruning weight measurements. Measurements were made in winter 2015.
3. Results and Discussion
3.1. Prototype Testing of Platform Robustness and Scanning Speed
3.2. Workflow Protocol Refinement: Plot Selection and Scan Cleaning Using Two Filters
- (1)
- Encoder dislodgement: Debris caught in the wheel sometimes dislodged the encoder, stopping it from spinning and tracking distance. This compressed the scan into two dimensions. There is no easy computational way of solving this problem, so the encoder was monitored during scans;
- (2)
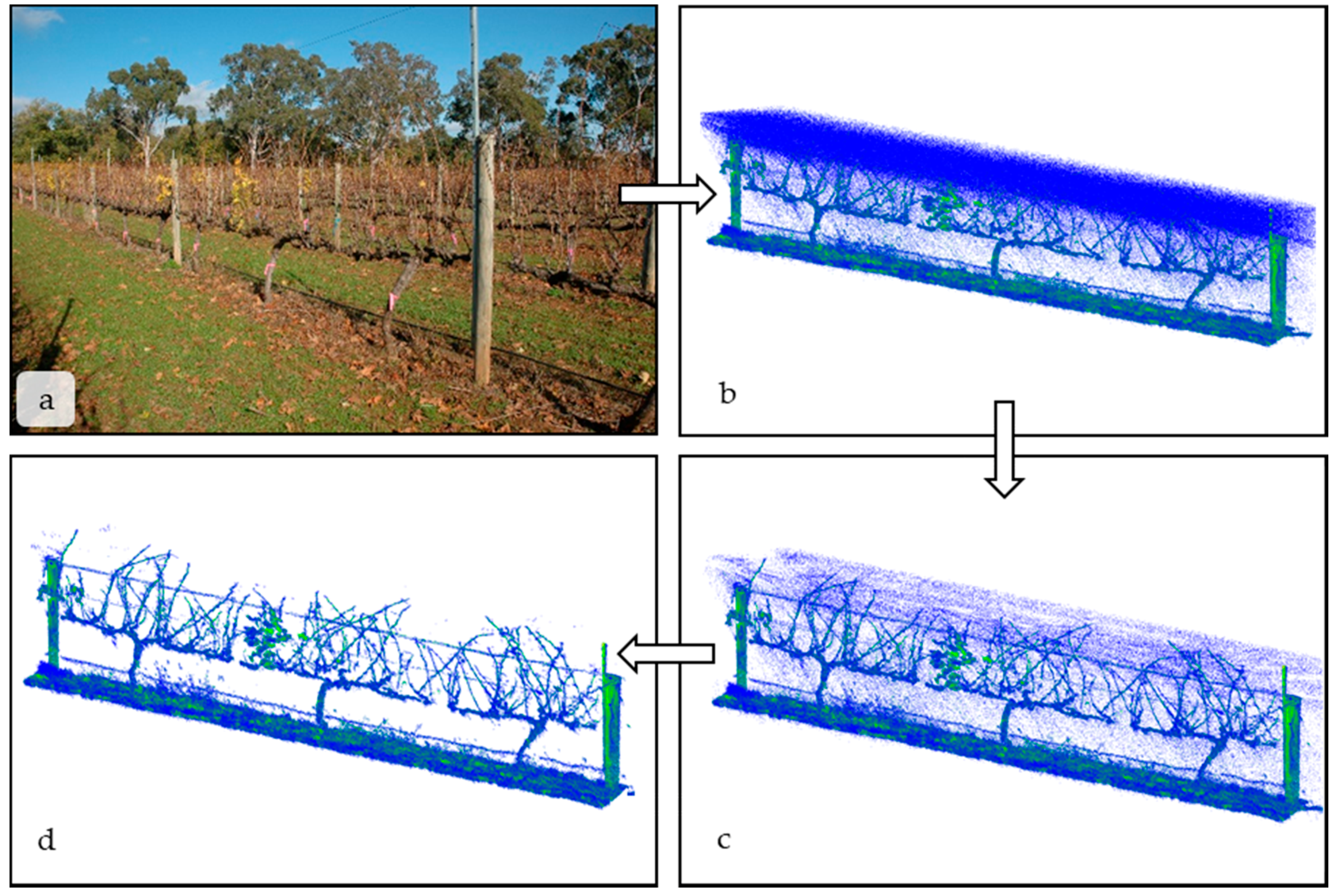
- Light intensity: Although the LMS-400 gives precise spatial and reflectance data at a high rate, it is designed to operate below 2000 Lx and not under high light conditions. Lx values in indirect sunlight commonly range between 1000 Lx on an overcast day to 130,000 Lx in direct sunlight [3]. High light levels are the cause of the spurious, low-intensity blue points seen between the LiDAR and the vines in Figure 2b. However, the erroneous measurements are all low reflectance values and can be removed by filtering the scan based on a set reflectance value. Points with reflectance values were removed from the scan using the “filter points by value” plugin in CloudCompare (Figure 2b,c). In Figure 2b, there are 1.49 million total points, and 28% of those points were . The reflectance threshold was chosen qualitatively and removed spurious points without significantly affecting the biological interpretation of the scan. Green leaf material had a reflectance value between one and five;
- (3)
- Edge scattering: As with any LiDAR scan, there was scattering at the edges of objects, where light is reflected in unpredictable ways. An example of edge scattering can be seen between the main vine cordon in Figure 2b. The nearest neighbor statistical outlier plugin in CloudCompare [27] removed sparse outliers based on the distance of an individual point from its neighbors. By applying these filters, point-clouds were reduced to only the scanned objects. In Figure 2c, there are 1.12 million points. After the filter removing statistical outliers is applied, there are 1.04 million points in the final point cloud (Figure 2d) on which any computational analysis would be performed.
3.3. LiDAR is Able to Capture Vine Size and Structure at All Growth Stages
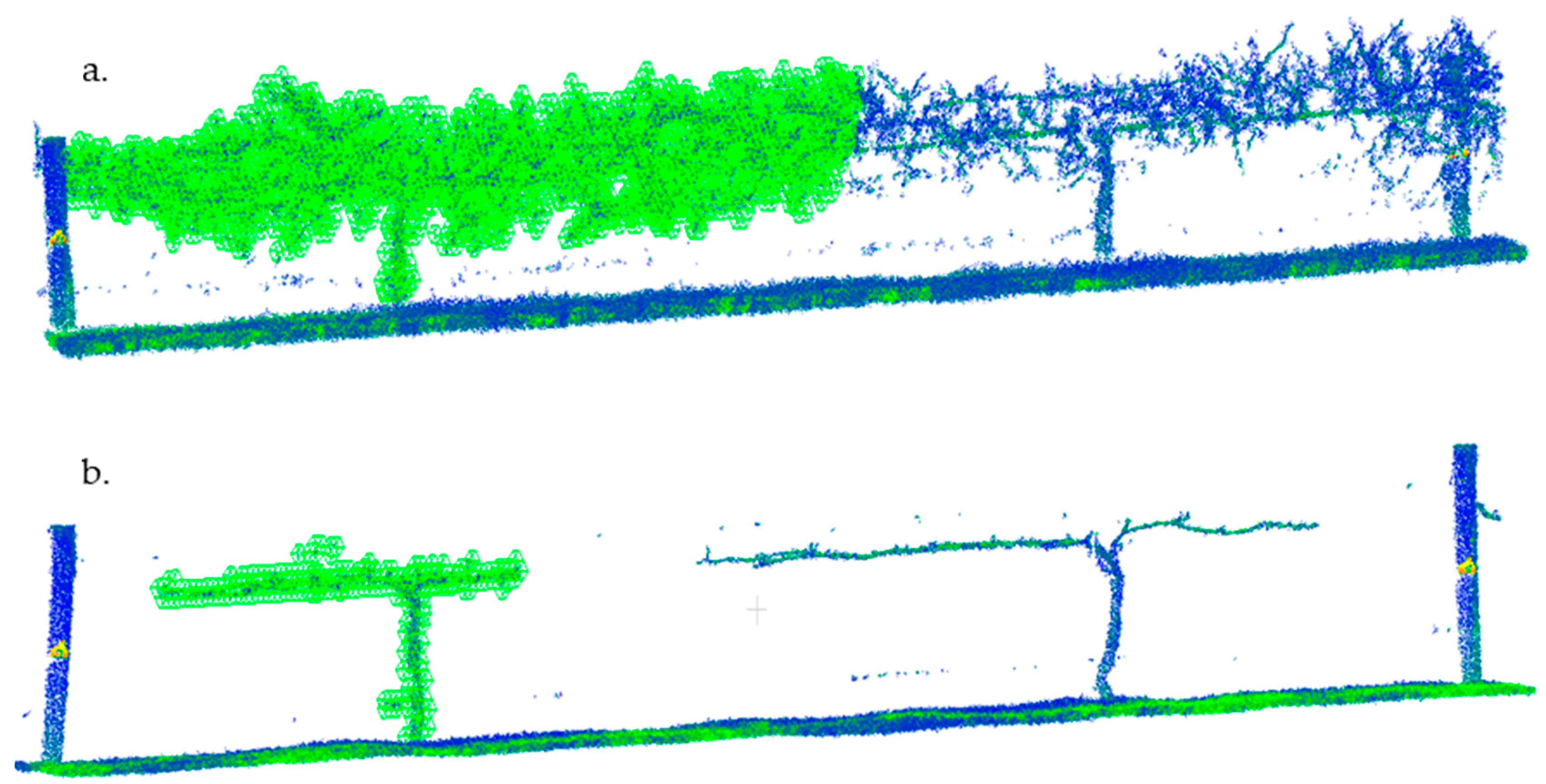
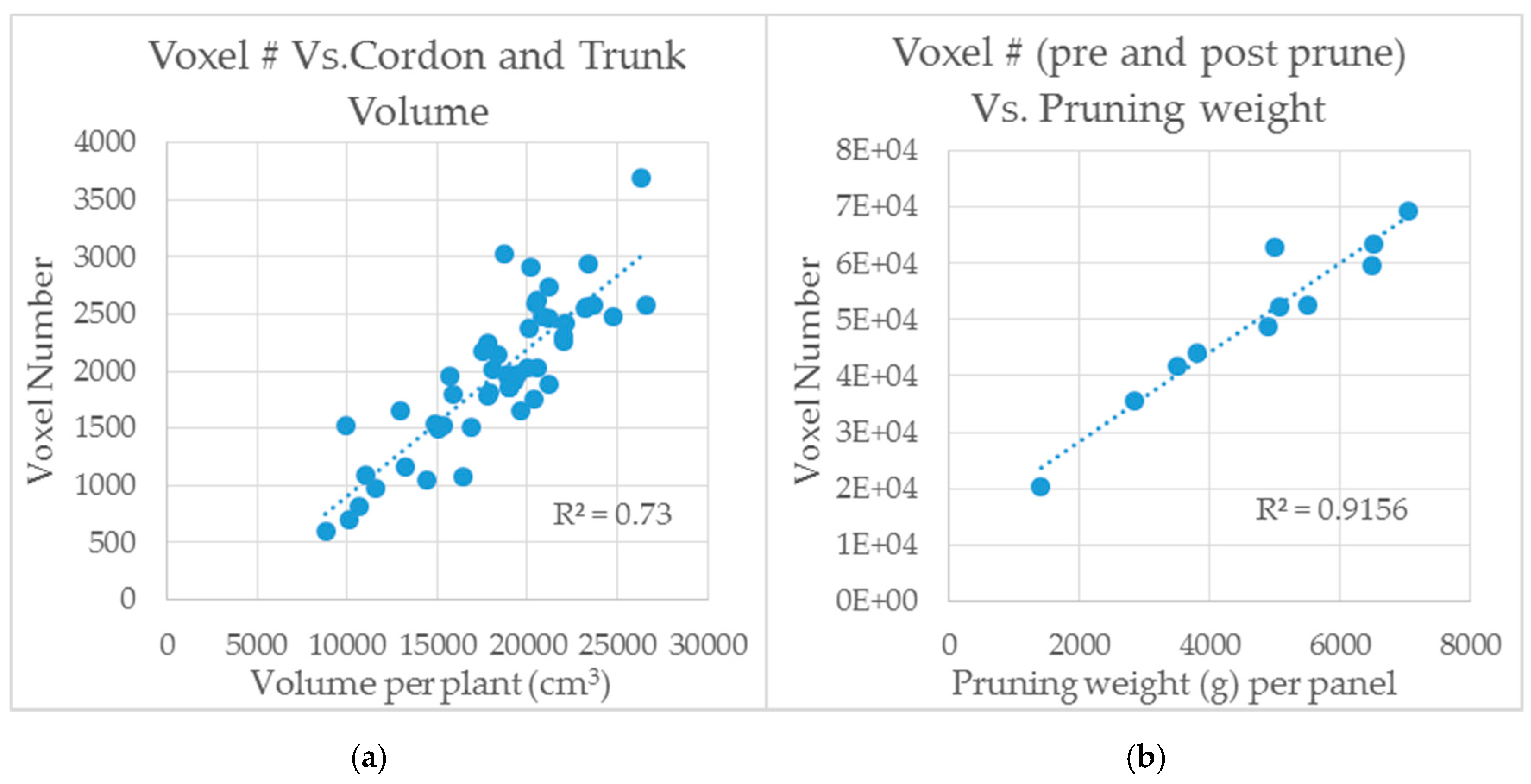
3.4. Preliminary Computational Analysis of LiDAR Scans by Voxelization Correlate with Measurements of Shiraz Trunk and Cordon Volume and Pruning Weights
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houle, D.; Govindaraju, D.R.; Omholt, S. Phenomics: The next challenge. Nat. Rev. Genet. 2010, 11, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.T.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Serbin, S.P.; Dillaway, D.N.; Kruger, E.L.; Townsend, P.A. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. J. Exp. Bot. 2012, 63, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Lamb, D.W.; Holzapfel, B.; Louis, J. Optical remote sensing applications in viticulture—A review. Aust. J. Grape Wine Res. 2002, 8, 36–47. [Google Scholar] [CrossRef]
- Johnson, L.F.; Roczen, D.E.; Youkhana, S.K.; Nemani, R.R.; Bosch, D.F. Mapping vineyard leaf area with multispectral satellite imagery. Comput. Electron. Agric. 2003, 38, 33–44. [Google Scholar] [CrossRef]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.B.; Ainsworth, E.A. High-Throughput Phenotyping of Maize Leaf Physiological and Biochemical Traits Using Hyperspectral Reflectance. Plant Physiol. 2017, 173, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal Remote Sensing Buggies and Potential Applications for Field-Based Phenotyping. Agronomy 2014, 4, 349–379. [Google Scholar] [CrossRef]
- Jimenez-Berni, J.A.; Deery, D.M.; Rozas-Larraondo, P.; Condon, A.T.G.; Rebetzke, G.J.; James, R.A.; Bovill, W.D.; Furbank, R.T.; Sirault, X.R.R. High Throughput Determination of Plant Height, Ground Cover, and Above-Ground Biomass in Wheat with LiDAR. Front. Plant Sci. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Bramley, R.G.V.; Trought, M.C.T.; Praat, J.-P. Vineyard variability in Marlborough, New Zealand: Characterising variation in vineyard performance and options for the implementation of Precision Viticulture. Aust. J. Grape Wine Res. 2011, 17, 72–78. [Google Scholar] [CrossRef]
- Bramley, R.G.V.; Siebert, T.E.; Herderich, M.J.; Krstic, M.P. Patterns of within-vineyard spatial variation in the “pepper” compound rotundone are temporally stable from year to year. Aust. J. Grape Wine Res. 2017, 23, 42–47. [Google Scholar] [CrossRef]
- Urretavizcaya, I.; Miranda, C.; Royo, J.B.; Santesteban, L.G. Within-vineyard zone delineation in an area with diversity of training systems and plant spacing using parameters of vegetative growth and crop load. In Precision Agriculture ’15; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 479–486. [Google Scholar]
- Arnó, J.; Escolà, A.; Vallès, J.M.; Llorens, J.; Sanz, R.; Masip, J.; Palacín, J.; Rosell-Polo, J.R. Leaf area index estimation in vineyards using a ground-based LiDAR scanner. Precis. Agric. 2013, 14, 290–306. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Cortiella, R.; Llorens-Calveras, J.; Escolà, A.; Arnó-Satorra, J.; Ribes-Dasi, M.; Masip-Vilalta, J.; Camp, F.; Gràcia-Aguilá, F.; Solanelles-Batlle, F.; Planas-DeMartí, S.; et al. Innovative LIDAR 3D Dynamic Measurement System to estimate fruit-tree leaf area. Sensors 2011, 11, 5769–5791. [Google Scholar] [CrossRef] [PubMed]
- Diago, M.P.; Krasnow, M.; Bubola, M.; Millan, B.; Tardaguila, J. Assessment of Vineyard Canopy Porosity Using Machine Vision. Am. J. Enol. Vitic. 2016, 67, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Meacham, K.; Sirault, X.; Quick, W.P.; von Caemmerer, S.; Furbank, R. Diurnal Solar Energy Conversion and Photoprotection in Rice Canopies. Plant Physiol. 2017, 173, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Rebetzke, G.J.; Reynolds, M. Integration of phenotyping and genetic platforms for a better understanding of wheat performance under drought. J. Exp. Bot. 2014, 65, 6167–6177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Qin, Z.; Liu, X.; Ustin, S.L. Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int. J. Appl. Earth Obs. Geoinf. 2003, 4, 295–310. [Google Scholar] [CrossRef]
- Lin, Y. LiDAR: An important tool for next-generation phenotyping technology of high potential for plant phenomics? Comput. Electron. Agric. 2015, 119, 61–73. [Google Scholar] [CrossRef]
- Schaefer, M.; Lamb, D. A Combination of Plant NDVI and LiDAR Measurements Improve the Estimation of Pasture Biomass in Tall Fescue (Festuca arundinacea var. Fletcher). Remote Sens. 2016, 8, 109. [Google Scholar] [CrossRef]
- Zolkos, S.G.; Goetz, S.J.; Dubayah, R. A meta-analysis of terrestrial aboveground biomass estimation using lidar remote sensing. Remote Sens. Environ. 2013, 128, 289–298. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Jimenez-Berni, J.A.; Bovill, W.D.; Deery, D.M.; James, R.A. High-throughput phenotyping technologies allow accurate selection of stay-green. J. Exp. Bot. 2016, 67, 4919–4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, A.N.; Gore, M.A.; Thompson, A. Cotton phenotyping with lidar from a track-mounted platform. In SPIE Commercial + Scientific Sensing and Imaging; International Society for Optics and Photonics: Bellingham, WA, USA, 2016. [Google Scholar]
- Wei, G.; Shalei, S.; Bo, Z.; Shuo, S.; Faquan, L.; Xuewu, C. Multi-wavelength canopy LiDAR for remote sensing of vegetation: Design and system performance. ISPRS J. Photogramm. Remote Sens. 2012, 69, 1–9. [Google Scholar] [CrossRef]
- Rosell Polo, J.R.; Sanz, R.; Llorens, J.; Arnó, J.; Escolà, A.; Ribes-Dasi, M.; Masip, J.; Camp, F.; Gràcia, F.; Solanelles, F.; et al. A tractor-mounted scanning LIDAR for the non-destructive measurement of vegetative volume and surface area of tree-row plantations: A comparison with conventional destructive measurements. Biosyst. Eng. 2009, 102, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Palleja, T.; Tresanchez, M.; Teixido, M.; Sanz, R.; Rosell, J.R.; Palacin, J. Sensitivity of tree volume measurement to trajectory errors from a terrestrial LIDAR scanner. Agric. For. Meteorol. 2010, 150, 1420–1427. [Google Scholar] [CrossRef]
- CloudCompare—Open Source Project. Available online: http://www.danielgm.net/cc/ (accessed on 16 March 2017).
- Rosu, R.B.; Marton, Z.C.; Blodow, N.; Dolha, M.; Beetz, M. Towards 3D Point cloud based object maps for household environments. Rob. Auton. Syst. 2008, 56, 927–941. [Google Scholar] [CrossRef]
- White, J.W.; Andrade-Sanchez, P.; Gore, M.A.; Bronson, K.F.; Coffelt, T.A.; Conley, M.M.; Feldmann, K.A.; French, A.N.; Heun, J.T.; Hunsaker, D.J.; et al. Field-based phenomics for plant genetics research. Field Crops Res. 2012, 133, 101–112. [Google Scholar] [CrossRef]
- Naito, H.; Ogawa, S.; Valencia, M.O.; Mohri, H.; Urano, Y.; Hosoi, F.; Shimizu, Y.; Chavez, A.L.; Ishitani, M.; Selvaraj, M.G.; et al. Estimating rice yield related traits and quantitative trait loci analysis under different nitrogen treatments using a simple tower-based field phenotyping system with modified single-lens reflex cameras. ISPRS J. Photogramm. Remote Sens. 2017, 125, 50–62. [Google Scholar] [CrossRef]
- Llorens, J.; Gil, E.; Llop, J.; Escolà, A. Variable rate dosing in precision viticulture: Use of electronic devices to improve application efficiency. Crop Prot. 2010, 29, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Gil, E.; Llorens, J.; Llop, J.; Fàbregas, X.; Escolà, A.; Rosell-Polo, J.R. Variable rate sprayer. Part 2—Vineyard prototype: Design, implementation, and validation. Comput. Electron. Agric. 2013, 95, 136–150. [Google Scholar] [CrossRef]
- Saeys, W.; Lenaerts, B.; Craessaerts, G.; De Baerdemaeker, J. Estimation of the crop density of small grains using LiDAR sensors. Biosyst. Eng. 2009, 102, 22–30. [Google Scholar] [CrossRef]
- Tandon, P.S.; Saelens, B.E.; Zhou, C.; Kerr, J.; Christakis, D.A. Indoor versus outdoor time in preschoolers at child care. Am. J. Prev. Med. 2013, 44, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Dry, P.R. Canopy management for fruitfulness. Aust. J. Grape Wine Res. 2000, 6, 109–115. [Google Scholar] [CrossRef]
- Perez-Sanz, F.; Navarro, P.J.; Egea-Cortines, M. Plant phenomics: An overview of image acquisition technologies and image data analysis algorithms. Gigascience 2017, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tagarakis, A.; Liakos, V.; Chatzinikos, T.; Koundouras, S.; Fountas, S.; Gemtos, T. Using laser scanner to map pruning wood in vineyards. In Precision Agriculture ’13; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 633–639. [Google Scholar]
- Liu, S.; Marden, S.; Whitty, M. Towards automated yield estimation in viticulture. In Proceedings of the Australasian Conference on Robotics and Automation, Sydney, Australia, 2–4 December 2013; Volume 24. [Google Scholar]
- Hall, A.; Quirk, L.; Wilson, M.; Hardie, J. Increasing the efficiency of forecasting winegrape yield by using information on spatial variability to select sample sites. In The Grapevine Management Guide 2009–2010; National Wine and Grape Industry Centre: Wagga Wagga, Australia.
- Dunn, G. Yield Forecasting. Available online: https://www.wineaustralia.com/getmedia/5304c16d-23b3-4a6f-ad53-b3d4419cc979/201006_Yield-Forecasting.pdf (accessed on 28 August 2018).

| Recursion Level (R) | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|
| R2 (Pruning weight) | 0.088 | 0.47 | 0.71 | 0.86 | 0.92 | 0.78 |
| R2 (Trunk & Cordon volume) | 0.25 | 0.59 | 0.65 | 0.73 | 0.73 | 0.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebers, M.H.; Edwards, E.J.; Jimenez-Berni, J.A.; Thomas, M.R.; Salim, M.; Walker, R.R. Fast Phenomics in Vineyards: Development of GRover, the Grapevine Rover, and LiDAR for Assessing Grapevine Traits in the Field. Sensors 2018, 18, 2924. https://doi.org/10.3390/s18092924
Siebers MH, Edwards EJ, Jimenez-Berni JA, Thomas MR, Salim M, Walker RR. Fast Phenomics in Vineyards: Development of GRover, the Grapevine Rover, and LiDAR for Assessing Grapevine Traits in the Field. Sensors. 2018; 18(9):2924. https://doi.org/10.3390/s18092924
Chicago/Turabian StyleSiebers, Matthew H., Everard J. Edwards, Jose A. Jimenez-Berni, Mark R. Thomas, Michael Salim, and Rob R. Walker. 2018. "Fast Phenomics in Vineyards: Development of GRover, the Grapevine Rover, and LiDAR for Assessing Grapevine Traits in the Field" Sensors 18, no. 9: 2924. https://doi.org/10.3390/s18092924





