Remote Blood Glucose Monitoring in mHealth Scenarios: A Review
Abstract
:1. Introduction
2. The Evolution of the Architectures for Remote Monitoring
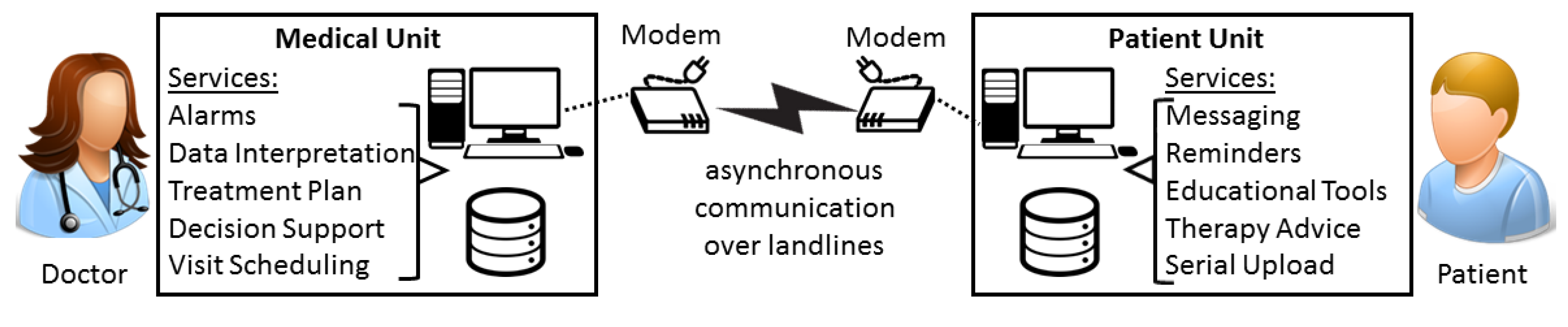
2.1. The Early Remote Monitoring Architecture Adopted for Diabetes Patients (1990–2000)
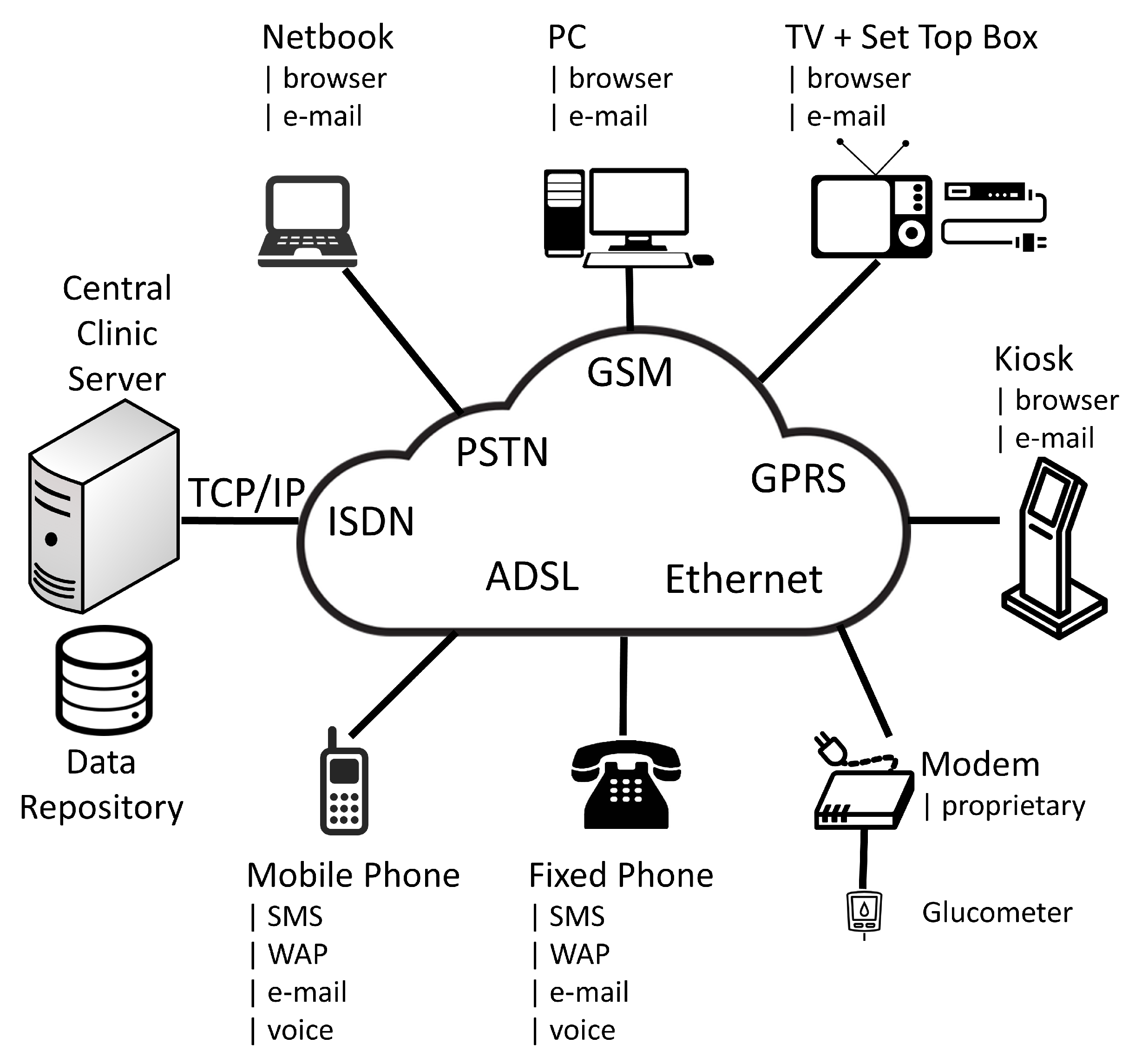
2.2. The Switch to a Service Oriented Architecture (2000–2010)
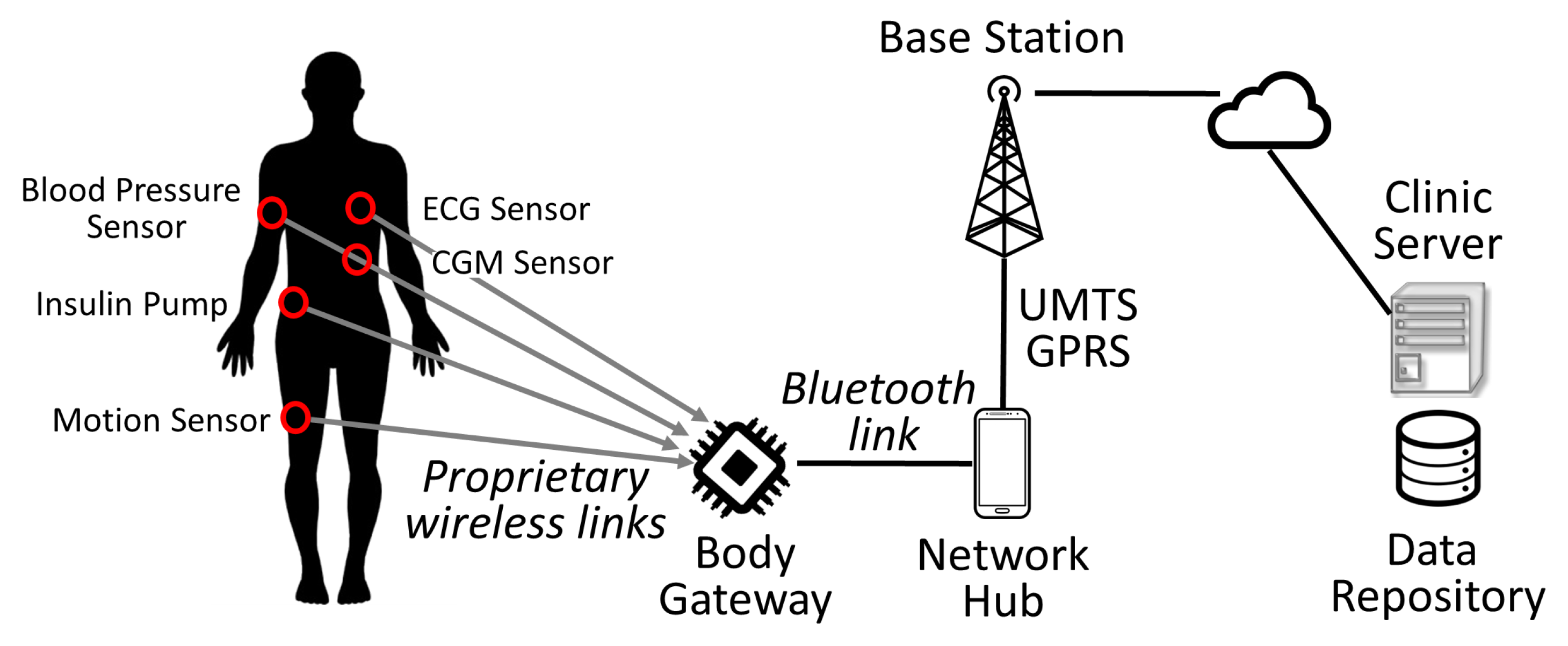
2.3. The Evolution to a Body Area Network Architecture (2010–Present)
3. Sending Data from Diabetes Devices to the Cloud
4. Our Experiences with Telemedicine Systems
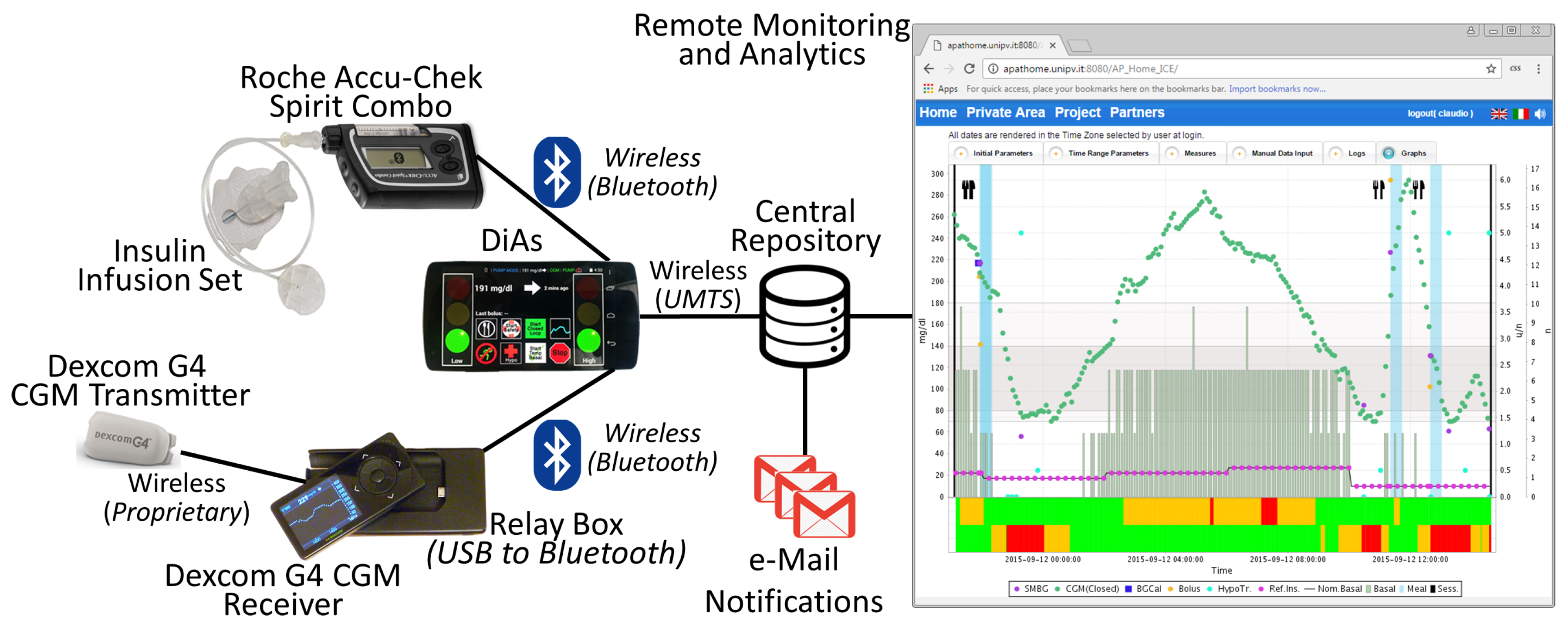
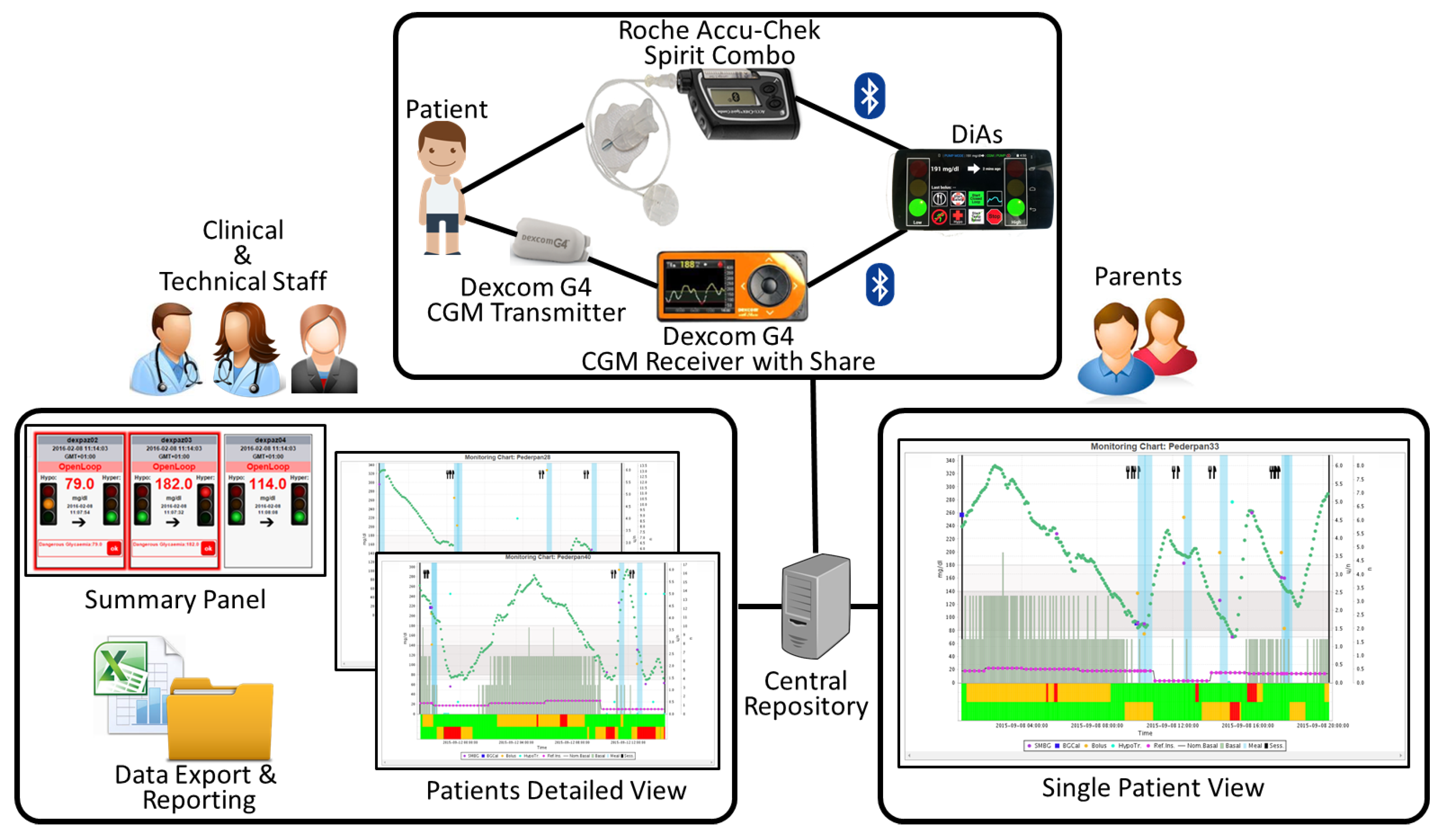
4.1. Artificial Pancreas in Adults
4.2. Artificial Pancreas in Adolescents
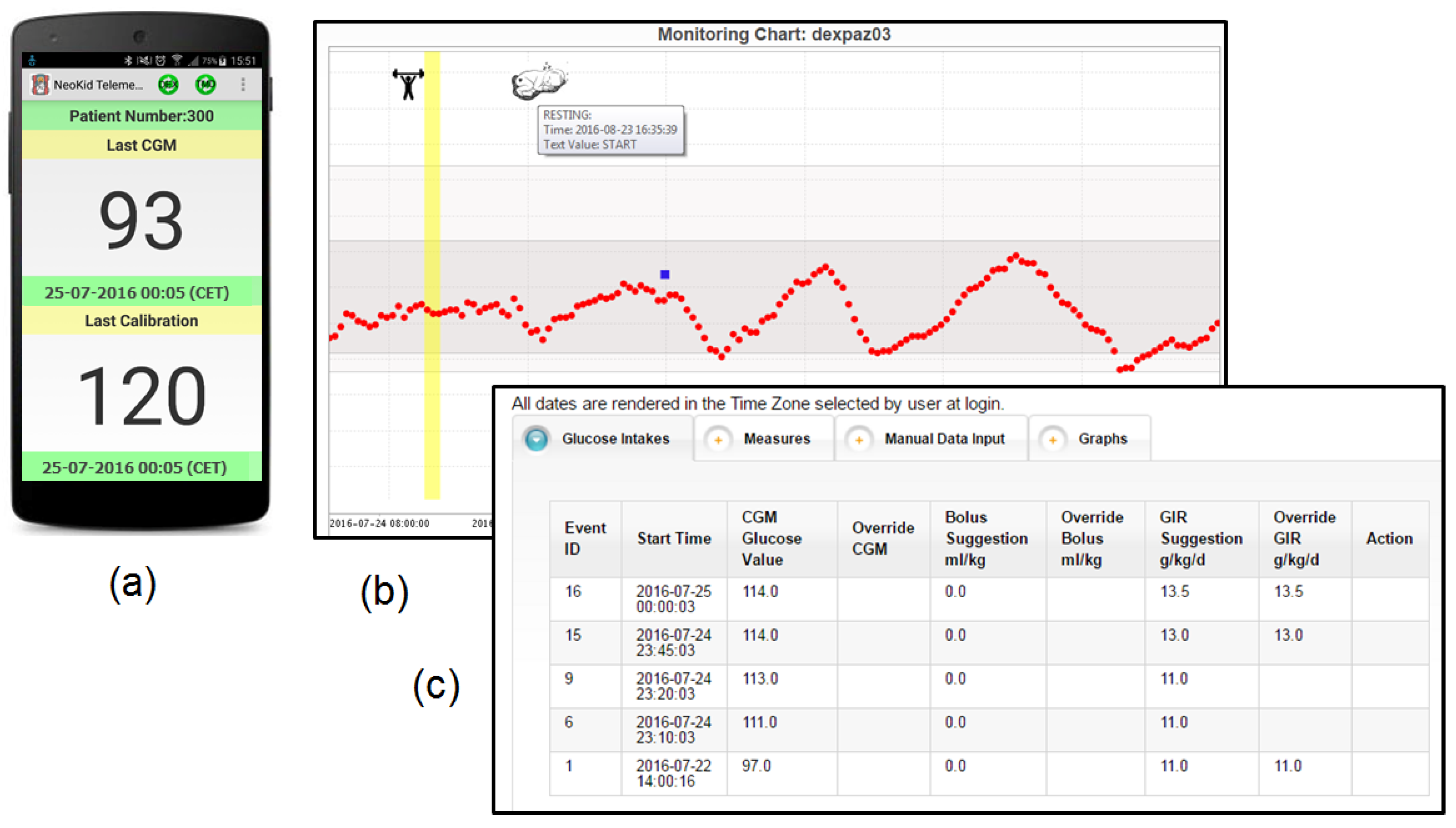
4.3. Neonatal Intensive Care Unit
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3G | Third Generation Mobile Communication System |
| AP | Artificial Pancreas |
| BAN | Body Area Network |
| BGL | Blood Glucose Level |
| CGM | Continuous Glucose Monitoring |
| DiAs | Diabetes Assistant |
| DTS | Diabetes Technology Society |
| DTSec | Diabetes Technology Society Security Standard for Connected Diabetes Devices |
| GD | Gestational Diabetes Mellitus |
| ICT | Information and Communication Technology |
| MU | Medical Unit |
| NICU | Neonatal Intensive Care Unit |
| PC | Personal Computer |
| PDA | Personal Digital Assistant |
| PU | Patient Unit |
| SMS | Short Message Service |
| SOA | Service Oriented Architecture |
| T1D | Type 1 Diabetes Mellitus |
| T2D | Type 2 Diabetes Mellitus |
| UMTS | Universal Mobile Telecommunications System |
| USB | Universal Serial Bus |
| WAP | Wireless Application Protocol |
| TV | Television |
References
- Bentham, J.; Danaei, G.; Di Cesare, M.; Ezzati, M.; Hajifathalian, K.; Kontis, V.; Lu, Y.; Zhou, B. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; Available online: http://www.who.int/diabetes/global-report/en (accessed on 11 November 2016).
- Kasper, D.; Fauci, A.; Hauser, S.; Longo, D.; Jameson, J.; Loscalzo, J. Harrison’s Principles of Internal Medicine; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Van Belle, T.; Coppieters, K.; von Herrath, M. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2010, 204, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet. Med. 2012, 29, 844–854. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Sparacino, G.; Zanon, M.; Facchinetti, A.; Zecchin, C.; Maran, A.; Cobelli, C. Italian Contributions to the Development of Continuous Glucose Monitoring Sensors for Diabetes Management. Sensors 2012, 12, 13753–13780. [Google Scholar] [CrossRef] [PubMed]
- Joubert, M.; Reznik, Y. Personal continuous glucose monitoring (CGM) in diabetes management: Review of the literature and implementation for practical use. Diabet. Res. Clin. Pract. 2012, 96, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Available online: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ClinicalChemistryandClinicalToxicologyDevicesPanel/UCM513025.pdf (accessed on 11 November 2016).
- McKinlay, C.J.; Alsweiler, J.M.; Ansell, J.M.; Anstice, N.S.; Chase, J.G.; Gamble, G.D.; Harris, D.L.; Jacobs, R.J.; Jiang, Y.; Paudel, N.; et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N. Engl. J. Med. 2015, 373, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.; Smith, E.; Sunehag, A. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics 2006, 118, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, G.; Skiold, B.; Karlen, J.; Tessma, M.; Norman, M.; Aden, U.; Vanpee, M. Early hyperglycemia is a risk factor for death and white matter reduction in preterm infants. Pediatrics 2010, 125, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.; Gabbay, R. Web-based management of diabetes through glucose uploads: Has the time come for telemedicine? Diabetes Res. Clin. Pract. 2009, 83, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, D.; Lanzola, G.; on behalf of the AP@home Consortium. Utilizing information technologies for lifelong monitoring in diabetes patients. J. Diabetes Sci. Technol. 2011, 5, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bellazzi, R.; Larizza, C.; Montani, S.; Riva, A.; Stefanelli, M.; D’Annunzio, G.; Lorini, R.; Gomez, E.; Hernando, E.; Brugues, E.; et al. A telemedicine support for diabetes management: The T-IDDM project. Comput. Method Progr. Biomed. 2002, 69, 147–161. [Google Scholar] [CrossRef]
- Falasconi, S.; Lanzola, G.; Stefanelli, M. An ontology-based multi-agent architecture for distributed health-care information systems. Methods Inf. Med. 1997, 36, 20–29. [Google Scholar] [PubMed]
- Riva, A.; Bellazzi, R.; Lanzola, G.; Stefanelli, M. A development environment for knowledge-based medical applications on the world-wide web. Artif. Intell. Med. 1998, 14, 279–293. [Google Scholar] [CrossRef]
- Klonoff, D.; True, M. The Missing Element of Telemedicine for Diabetes: Decision Support Software. J. Diabetes Sci. Technol. 2009, 3, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Hernando, M.; Garcıa, A.; Del Pozo, F.; Cermeno, J.; Corcoy, R.; Brugues, E.; De Leiva, A. Telemedicine as a tool for intensive management of diabetes: The DIABTel experience. Comput. Methods Progr. Biomed. 2002, 69, 163–177. [Google Scholar] [CrossRef]
- Lanzola, G.; Capozzi, D.; D’Annunzio, G.; Ferrari, P.; Bellazzi, R.; Larizza, C. Going mobile with a multiaccess service for the management of diabetic patients. J. Diabetes Sci. Technol. 2007, 1, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.; Pascual, M.; Salvador, C.; Garcia-Saez, G.; Rodriguez-Herrero, A.; Martinez-Sarriegui, I.; Gomez, E. Definition of Information Technology Architectures for Continuous Data Management and Medical Device Integration in Diabetes. J. Diabetes Sci. Technol. 2008, 2, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, G.; Hernando, M.; Martinez-Sarriegui, I.; Rigla, M.; Torralba, V.; Brugues, E.; de Leiva, A.; Gomez, E. Architecture of a wireless Personal Assistant for telemedical diabetes care. Int. J. Med. Inform. 2009, 78, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Franc, S.; Dardari, D.; Boucherie, B.; Riveline, J.; Biedzinski, M.; Petit, C.; Requeda, E.; Leurent, P.; Varroud-Vial, M.; Hochberg, G.; et al. Real-life application and validation of flexible intensive insulin-therapy algorithms in type 1 diabetes patients. Diabetes Metab. 2009, 35, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Larizza, C.; Bellazzi, R.; Stefanelli, M.; Ferrari, P.; De Cata, P.; Gazzaruso, C.; Fratino, P.; D’Annunzio, G.; Hernando, E.; Gomez, E. The M2DM Project—The experience of two Italian clinical sites with clinical evaluation of a multi-access service for the management of diabetes mellitus patients. Methods Inf. Med. 2006, 45, 79–84. [Google Scholar] [PubMed]
- Capozzi, D.; Lanzola, G. An Agent-Based Architecture for Home Care Monitoring and Education of Chronic Patients. In Proceedings of the IEEE Conference on Complexity in Engineering, Rome, Italy, 22–24 February 2010; pp. 138–140.
- Capozzi, D.; Lanzola, G. A configurable home care platform for monitoring patients with reminder messaging and compliance tracking services. Stud. Health Technol. Inform. 2010, 160, 63–67. [Google Scholar] [PubMed]
- Yuce, M. Implementation of wireless body area networks for healthcare systems. Sens. Actuators A Phys. 2010, 162, 116–129. [Google Scholar] [CrossRef]
- Ettelt, S.; Nolte, E.; McKee, M.; Haugen, O.; Karlberg, I.; Klazinga, N.; Ricciardi, W.; Teperi, J. Evidence-based policy? The use of mobile phones in hospital. J. Public Health 2006, 28, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Young, G. Remote Monitoring for Implantable Cardiac Electronic Devices. Heart Lung Circ. 2012, 21, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, S.; Devanand, M. Remote monitoring for diabetes disorder: Pilot study using InDiaTel prototype. Eur. Res. Telemed. 2015, 4, 63–69. [Google Scholar] [CrossRef]
- Keith-Hynes, P.; Mize, B.; Robert, A.; Place, J. The Diabetes Assistant: A Smartphone-Based System for Real-Time Control of Blood Glucose. Electronics 2014, 3, 609–623. [Google Scholar] [CrossRef]
- Place, J.; Robert, A.; Brahim, N.B.; Keith-Hynes, P.; Farret, A.; Pelletier, M.; Buckingham, B.; Breton, M.; Kovatchev, B.; Renard, E. DiAs web monitoring: A real-time remote monitoring system designed for artificial pancreas outpatient trials. J. Diabetes Sci. Technol. 2013, 7, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Foster, J. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [PubMed]
- Bergenstal, R.; Anderson, R.; Bina, D.; Johnson, M.; Davidson, J.; Solarz-Johnson, B.; Kendall, D. Impact of modem-transferred blood glucose data on clinician work efficiency and patient glycemic control. Diabetes Technol. Ther. 2005, 7, 241–247. [Google Scholar] [CrossRef] [PubMed]
- The Nightscout Project. Available online: http://www.nightscout.info (accessed on 18 November 2016).
- The Nightscout Foundation. Available online: http://www.nightscoutfoundation.org (accessed on 18 November 2016).
- Cobelli, C.; Renard, E.; Kovatchev, B. The artificial pancreas: A digital-age treatment for diabetes. Lancet Diabetes Endocrinol. 2014, 2, 679–681. [Google Scholar] [CrossRef]
- Heinemann, L.; Benesch, C.; DeVries, J.; on behalf of the AP@home Consortium. AP@home: The Artificial Pancreas is Now at Home. J. Diabetes Sci. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Luijf, Y.; DeVries, J.; Zwinderman, K.; Leelarathna, L.; Nodale, M.; Caldwell, K.; Kumareswaran, K.; Elleri, D.; Allen, J.; Wilinska, M.; et al. Day and night closed-loop control in adults with type 1 diabetes a comparison of two closed-loop algorithms driving continuous subcutaneous insulin infusion versus patient self-management. Diabetes Care 2013, 36, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Lanzola, G.; Capozzi, D.; Serina, N.; Magni, L.; Bellazzi, R.; on behalf of the AP@home Consortium. Bringing the artificial pancreas home: Telemedicine aspects. J. Diabetes Sci. Technol. 2011, 5, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, D.; Lanzola, G. A generic telemedicine infrastructure for monitoring an artificial pancreas trial. Comput. Methods Progr. Biomed. 2013, 110, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, S.; Bruttomesso, D.; Di Palma, F.; Lanzola, G.; Visentin, R.; Filippi, A.; Scotton, R.; Toffanin, C.; Messori, M.; Scarpellini, S.; et al. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care 2014, 37, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, S.; Place, J.; Kropff, J.; Messori, M.; Keith-Hynes, P.; Visentin, R.; Monaro, M.; Galasso, S.; Boscari, F.; Toffanin, C.; et al. Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes. Metab. 2015, 17, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Lanzola, G.; Scarpellini, S.; Di Palma, F.; Toffanin, C.; Del Favero, S.; Magni, L.; Bellazzi, R.; on behalf of the AP@home Consortium. Monitoring Artificial Pancreas Trials Through Agent-based Technologies: A Case Report. J. Diabetes Sci. Technol. 2014, 8, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Lanzola, G.; Toffanin, C.; Di Palma, F.; Del Favero, S.; Magni, L.; Bellazzi, R.; on behalf of the AP@home Consortium. Designing an artificial pancreas architecture: The AP@home experience. Med. Biol. Eng. Comput. 2015, 53, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Kropff, J.; Del Favero, S.; Place, J.; Toffanin, C.; Visentin, R.; Monaro, M.; Messori, M.; Di Palma, F.; Lanzola, G.; Farret, A.; et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: A randomised crossover trial. Lancet Diabetes Endocrinol. 2015, 3, 939–947. [Google Scholar] [CrossRef]
- Renard, E.; Farret, A.; Kropff, J.; Bruttomesso, D.; Messori, M.; Place, J.; Visentin, R.; Calore, R.; Toffanin, C.; Di Palma, F.; et al. Day-and-Night Closed-Loop Glucose Control in Patients With Type 1 Diabetes Under Free-Living Conditions: Results of a Single-Arm 1-Month Experience Compared With a Previously Reported Feasibility Study of Evening and Night at Home. Diabetes Care 2016, 39, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Zisser, H.; Renard, E.; Kovatchev, B.; Cobelli, C.; Avogaro, A.; Nimri, R.; Magni, L.; Buckingham, B.; Chase, H.; Doyle, F.; et al. Multicenter closed-loop insulin delivery study points to challenges for keeping blood glucose in a safe range by a control algorithm in adults and adolescents with type 1 diabetes from various sites. Diabetes Technol. Ther. 2014, 16, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wadams, H.; Cherñavvsky, D.R.; Lteif, A.; Basu, A.; Kovatchev, B.P.; Kudva, Y.C.; DeBoer, M.D. Closed-loop control for pediatric Type 1 diabetes mellitus. Diabetes Manag. 2015, 5, 25–35. [Google Scholar] [CrossRef]
- Del Favero, S.; Boscari, F.; Messori, M.; Rabbone, I.; Bonfanti, R.; Sabbion, A.; Iafusco, D.; Schiaffini, R.; Visentin, R.; Calore, R.; et al. Randomized summer camp crossover trial in 5-to 9-year-old children: Outpatient wearable artificial pancreas is feasible and safe. Diabetes Care 2016. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, V.; Becker, D.; Escobar, O.; Siminerio, L. Families with children with diabetes: Implications of parent stress for parent and child health. J. Pediatr. Psychol. 2012, 37, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, K.; Deiss, D.; Buckingham, B.; Cantwell, M.; Kache, S.; Agarwal, S.; Wilson, D.; Steil, G. Glucose Control in Pediatric Intensive Care Unit Patients Using an Insulin–Glucose Algorithm. Diabetes Technol. Ther. 2007, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Steil, G.; Deiss, D.; Shih, J.; Buckingham, B.; Weinzimer, S.; Agus, M. Intensive Care Unit Insulin Delivery Algorithms: Why So Many? How to Choose? J. Diabetes Sci. Technol. 2009, 3, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Pardeep, K.; Hoon-Jae, L. Security Issues in Healthcare Applications Using Wireless Medical Sensor Networks: A Survey. Sensors 2012, 12, 55–91. [Google Scholar]
- Paul, N.; Kohno, T.; Klonoff, D. A Review of the Security of Insulin Pump Infusion Systems. J. Diabetes Sci. Technol. 2011, 5, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Technology Society. DTS Cybersecurity Standard for Connected Diabetes Devices. Available online: http://www.diabetestechnology.org/dtsec.shtml (accessed on 11 November 2016).
- Klaassen, B.; van Beijnum, B.; Hermens, H. Usability in telemedicine systems—A literature survey. Int. J. Med. Inform. 2016, 93, 57–69. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, N.; Schumann, M.; Kraft, K.; Hoffmann, W. Telemedicine and telecare for older patients—A systematic review. Maturitas 2012, 73, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ren, B. The monitoring and managing application of cloud computing based on Internet of Things. Comput. Methods Progr. Biomed. 2016, 130, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.; Ghemawat, S. MapReduce: Simplified data processing on large clusters. Commun. ACM 2008, 51, 107–113. [Google Scholar] [CrossRef]
- Mezghani, E.; Exposito, E.; Drira, K.; Da Silveira, M.; Pruski, C. A Semantic Big Data Platform for Integrating Heterogeneous Wearable Data in Healthcare. J. Med. Syst. 2015, 39, 185. [Google Scholar] [CrossRef] [PubMed]
- Kahn, E.; La Marca, F.; Mazzola, C.A. Neurosurgery and Telemedicine in the United States: Assessment of the Risks and Opportunities. World Neurosurg. 2016, 89, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Oron, T.; Farfel, A.; Muller, I.; Miller, S.; Atlas, E.; Nimri, R.; Phillip, M. A remote monitoring system for artificial pancreas support is safe, reliable, and user friendly. Diabetes Technol. Ther. 2014, 16, 699–705. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzola, G.; Losiouk, E.; Del Favero, S.; Facchinetti, A.; Galderisi, A.; Quaglini, S.; Magni, L.; Cobelli, C. Remote Blood Glucose Monitoring in mHealth Scenarios: A Review. Sensors 2016, 16, 1983. https://doi.org/10.3390/s16121983
Lanzola G, Losiouk E, Del Favero S, Facchinetti A, Galderisi A, Quaglini S, Magni L, Cobelli C. Remote Blood Glucose Monitoring in mHealth Scenarios: A Review. Sensors. 2016; 16(12):1983. https://doi.org/10.3390/s16121983
Chicago/Turabian StyleLanzola, Giordano, Eleonora Losiouk, Simone Del Favero, Andrea Facchinetti, Alfonso Galderisi, Silvana Quaglini, Lalo Magni, and Claudio Cobelli. 2016. "Remote Blood Glucose Monitoring in mHealth Scenarios: A Review" Sensors 16, no. 12: 1983. https://doi.org/10.3390/s16121983





