Development of a Flexible Non-Metal Electrode for Cell Stimulation and Recording
Abstract
:1. Introduction
2. Literature Review
2.1. Non-Metallic Electrodes
2.2. Flexible Electrodes
2.3. Stimulation Applications of Electrodes
3. Characteristics of PDMS Material
4. Materials and Methods
4.1. Experimental Materials and Equipment
4.2. Design of the Flexible Electrodes
4.3. Manufacture of the Flexible Electrodes
4.4. Electrochemical Properties
4.5. Biocompatibility of the Electrode
5. Results and Discussions
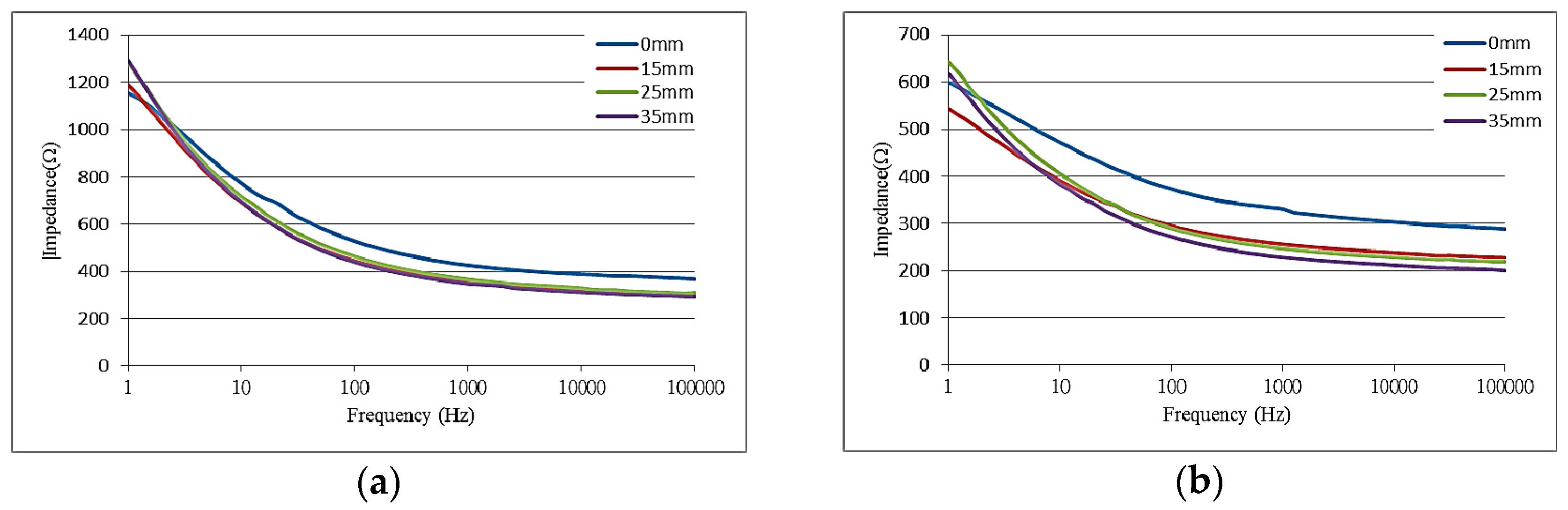
5.1. Electrochemical Properties of the Flexible Electrodes
5.2. Mechanical Properties of the Flexible Electrode
5.3. Biocompatibility of the Flexible Electrode
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Chou, N.; Byun, D.; Kim, S. MEMS-based microelectrode technologies capable of penetrating neural tissues. Biomed. Eng. Lett. 2014, 4, 109–119. [Google Scholar] [CrossRef]
- Kipke, D.R.; Vetter, R.J.; Williams, J.C.; Hetke, J.F. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Guzelian, A.A.; Agnew, W.F.; Yuen, T.G.; McCreery, D.B. Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. J. Neurosci. Methods 2004, 137, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-B.; Li, G.; Sun, X.-N.; Zhu, Z.-H.; Jin, Q.-H.; Zhao, J.-L.; Ren, Q.-S. Integration of Au nanorods with flexible thin-film microelectrode arrays for improved neural interfaces. J. Microelectr. Syst. 2009, 18, 88–96. [Google Scholar] [CrossRef]
- Suaning, G.; Lovell, N.; Kwok, C. Fabrication of platinum spherical electrodes in an intra-ocular prosthesis using high-energy electrical discharge. Sens. Actuators A Phys. 2003, 108, 155–161. [Google Scholar] [CrossRef]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Gasbarro, C.M.; Plante, T.D. The influence of electrolyte com position on the in vitro charge-injection limits of activated iridium oxide (AIROF) stimulation electrodes. J. Neural Eng. 2007, 4, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Kwon, G.H.; Kim, J.Y.; Lee, S.H.; Sun, K.; Lee, S.H. Stable deposition and patterning of metal layers on the PDMS substrate and characteriz-ation for the development of the flexible and implantable micro electrode. Solid State Phenom. 2007, 124–126, 165–168. [Google Scholar] [CrossRef]
- Kim, J.; Song, X.; Kinoshita, K.; Madou, M.; White, R. Electrochemical studies of carbon films from pyrolyzed photoresist. J. Electrochem. Soc. 1998, 145, 2314–2319. [Google Scholar] [CrossRef]
- Musameh, M.; Wang, J.; Merkoci, A.; Lin, Y. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochem. Commun. 2002, 4, 743–746. [Google Scholar] [CrossRef]
- Mroz, A. Disposable reference electrode. Analyst 1998, 123, 1373–1376. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Yoshimatsu, T.; Nishi, N. New class of Ag/AgCl electrodes based on hydrophobic ionic liquid saturated with AgCl. Anal. Chem. 2007, 79, 7187–7191. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Park, S.; Boo, H.; Kim, H.C.; Chung, T.D. Nanoporous platinum solid-state reference electrode with layer-by-layer polyelectrolyte junction for pH sensing chip. Lab Chip 2011, 11, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-J.; Maxwell, E.J.; Parolo, C.; Bwambok, D.K.; Subramaniam, A.B.; Whitesides, G.M. Paper-based electroanalytical devices with an integrated, stable reference electrode. Lab Chip 2013, 13, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Chou, N.; Kim, S. A fabrication method of out-of-plane stretchable and flexible electrodes based on PDMS. In Proceedings of the 2013 SPIE Micro/Nano Materials, Devices, and Systems, Melbourne, Australia, 8–11 December 2013.
- Guo, L.; DeWeerth, S.P. PDMS-based conformable microelectrode arrays with selectable novel 3-D microelectrode geometries for surface stimulation and recording. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009.
- Zhu, Z.-H.; Zhou, L.; Zhang, H.; Li, G.; Jin, Q.-H.; Zhao, J.-L. Fabrication of carbon film-based flexible neural microelectrode array. Nanotechnol. Precis. Eng. 2012, 2, 017. [Google Scholar]
- Da Silva, E.T.S.G.; Miserere, S.; Kubota, L.T.; Merkoçi, A. Simple on plastic/paper inkjet-printed solid-state Ag/AgCl pseudo-reference electrode. Anal. Chem. 2014, 86, 10531–10534. [Google Scholar] [CrossRef] [PubMed]
- Chou, N.; Yoo, S.; Kim, S. A largely deformable surface type neural electrode array based on PDMS. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Bhandari, R.; Solzbacher, F. Morphology and electrochemical properties of activated and sputtered iridium oxide films for functional electrostimulation. J. Sens. Technol. 2012, 2, 138–147. [Google Scholar] [CrossRef]
- Luo, X.; Weaver, C.L.; Zhou, D.D.; Greenberg, R.; Cui, X.T. Highly stable carbon nanotube doped poly(3, 4-ethylenedioxy-thiophene) for chronic neural stimulation. Biomaterials 2011, 32, 5551–5557. [Google Scholar] [CrossRef] [PubMed]
- Brugger, J.; Beljakovic, G.; Despont, M.; Biebuyck, H.; De Rooij, N.; Vettiger, P. Low-cost PDMS seal ring for single-side wet etching of MEMS structures. Sens. Actuators A Phys. 1998, 70, 191–194. [Google Scholar] [CrossRef]
- Lei, K.F.; Lee, K.-F.; Lee, M.-Y. Development of a flexible PDMS capacitive pressure sensor for plantar pressure measurement. Microelectron. Eng. 2012, 99, 1–5. [Google Scholar] [CrossRef]
- Luo, C.; Meng, F.; Francis, A. Fabrication and application of silicon-reinforced PDMS masters. J. Microelectron. 2006, 37, 1036–1046. [Google Scholar] [CrossRef]
- Luo, C.; Meng, F.; Liu, X.; Guo, Y. Reinforcement of a PDMS master using an oxide-coated silicon plate. J. Microelectron. 2006, 37, 5–11. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Heinze, J. Cyclic voltammetry—“Electrochemical spectroscopy”. New analytical methods (25). Angew. Chem. Int. Ed. Engl. 1984, 23, 831–847. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Q.; Bin, C.; Liang, G.-T.; Li, W.; Zhou, X.; Liu, D. Study on microenvironment acidification by microfluidic chip with multilayer-paper supported breast cancer tissue. Chin. J. Anal. Chem. 2013, 41, 822–827. [Google Scholar]
- Chow, A.Y.; Chow, V.Y. Subretinal electrical stimulation of the rabbit retina. Neurosci. Lett. 1997, 225, 13–16. [Google Scholar] [CrossRef]
- Brindley, G. The site of electrical excitation of the human eye. J. Phys. 1955, 127, 189–200. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Howley, E.T. Exercise Physiology: Theory and Application to Fitness and Performance, 9th ed.; McGraw-Hill: Cleveland, OH, USA, 2013. [Google Scholar]





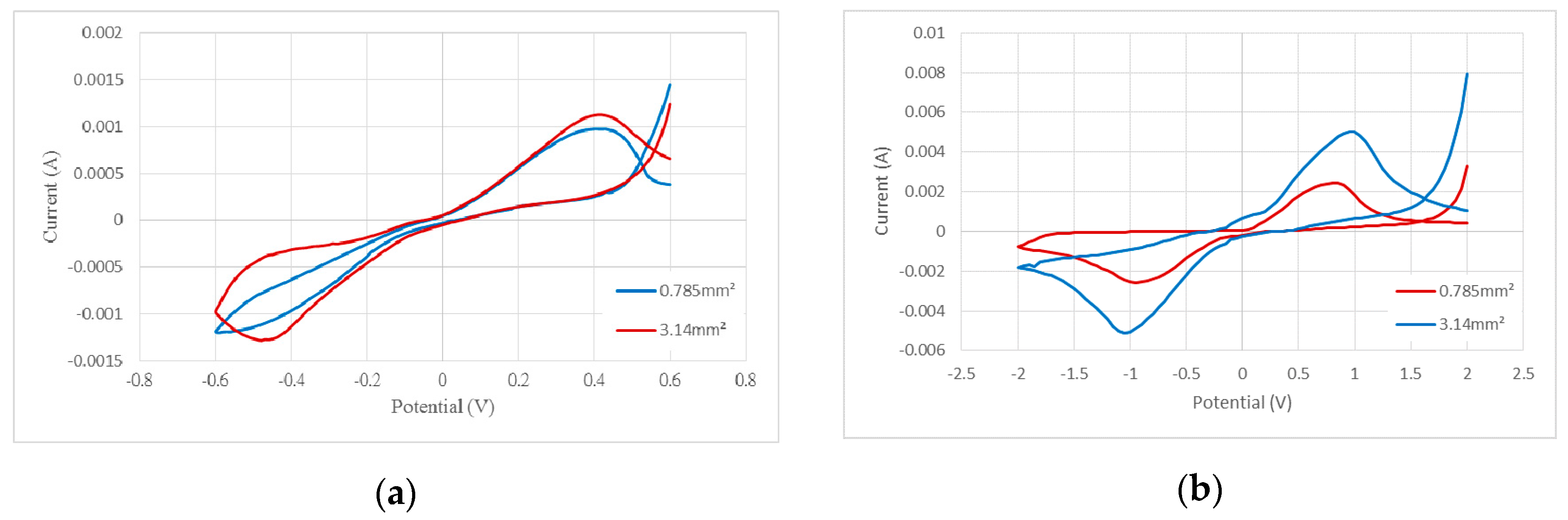



| Voltage Range/Area | 0.785 mm2 | 3.14 mm2 |
|---|---|---|
| ±0.6 V | 138.16 | 61.57 |
| ±2 V | 86.98 | 54.14 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.-S.A.; Syu, W.-J.; Lei, K.F.; Hwang, Y.-S. Development of a Flexible Non-Metal Electrode for Cell Stimulation and Recording. Sensors 2016, 16, 1613. https://doi.org/10.3390/s16101613
Gong C-SA, Syu W-J, Lei KF, Hwang Y-S. Development of a Flexible Non-Metal Electrode for Cell Stimulation and Recording. Sensors. 2016; 16(10):1613. https://doi.org/10.3390/s16101613
Chicago/Turabian StyleGong, Cihun-Siyong Alex, Wun-Jia Syu, Kin Fong Lei, and Yih-Shiou Hwang. 2016. "Development of a Flexible Non-Metal Electrode for Cell Stimulation and Recording" Sensors 16, no. 10: 1613. https://doi.org/10.3390/s16101613






