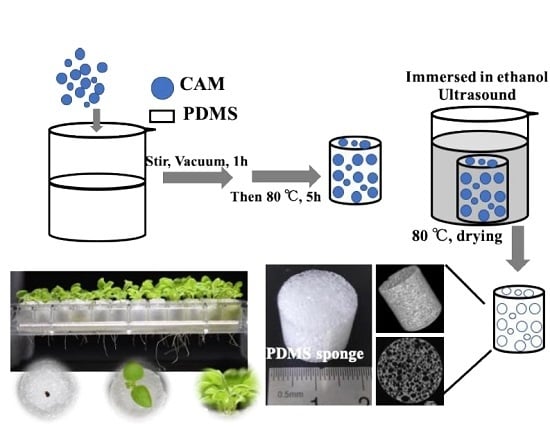
Hydrophilic Porous Polydimethysiloxane Sponge as a Novel 3D Matrix Mimicking Heterogeneous Pores in Soil for Plant Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
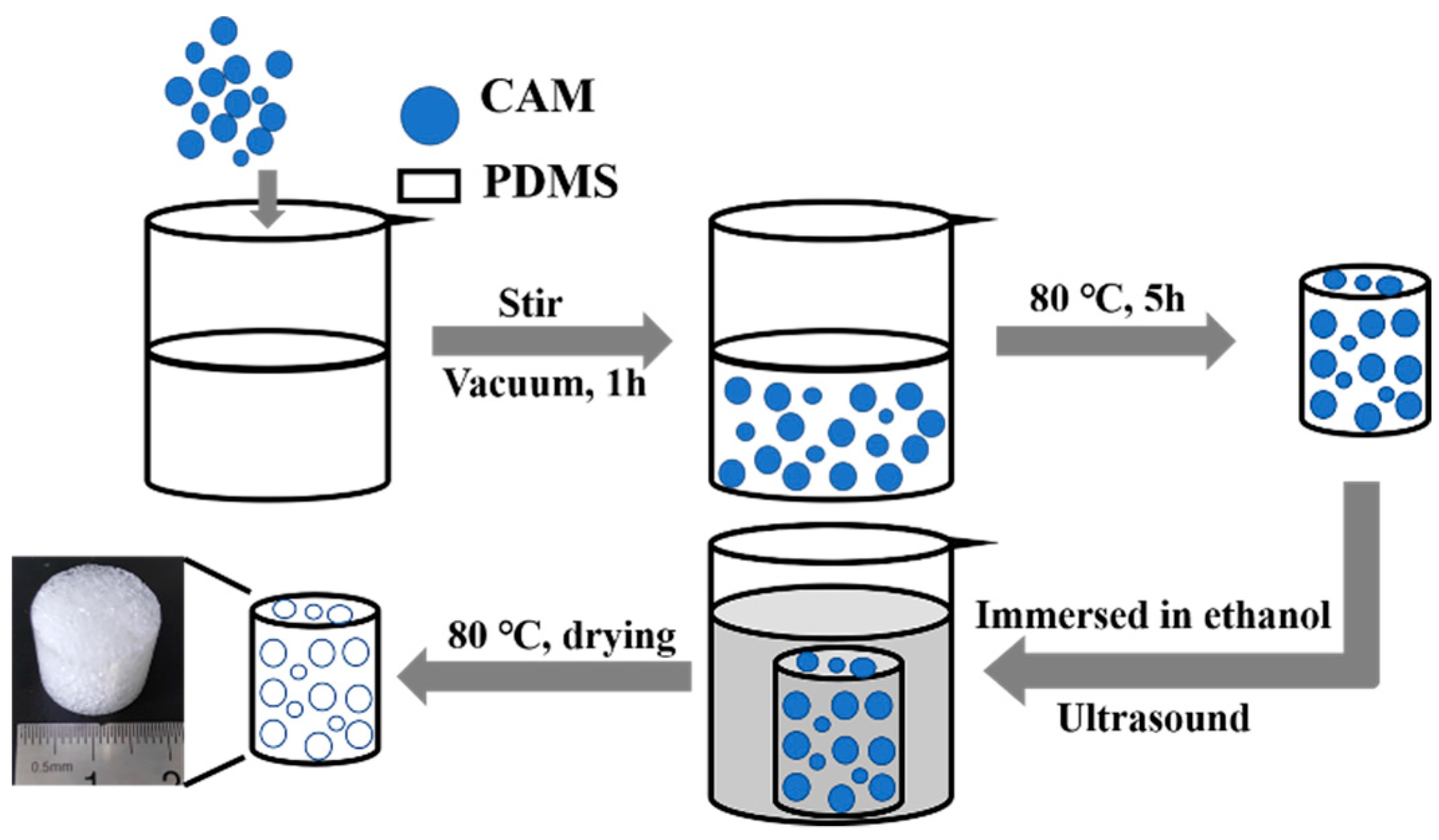
2.2. Preparation of Porous PDMS Sponge
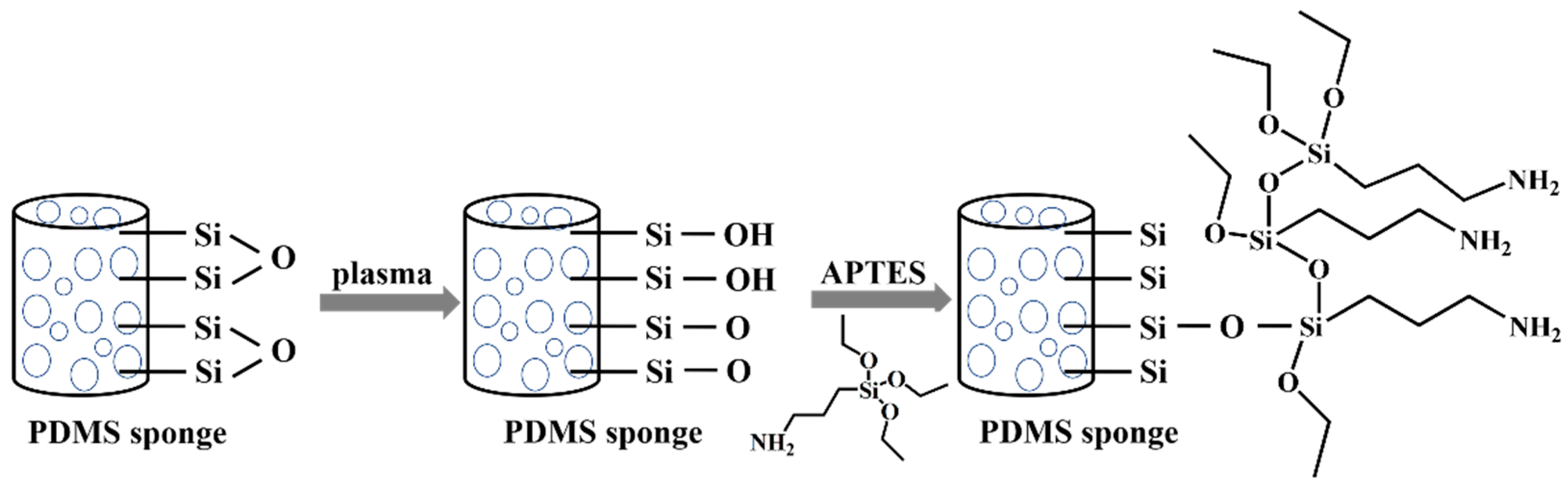
2.3. Characterization of the PDMS Sponge
2.4. Plant Cultivation on PDMS Sponges
3. Results and Discussion
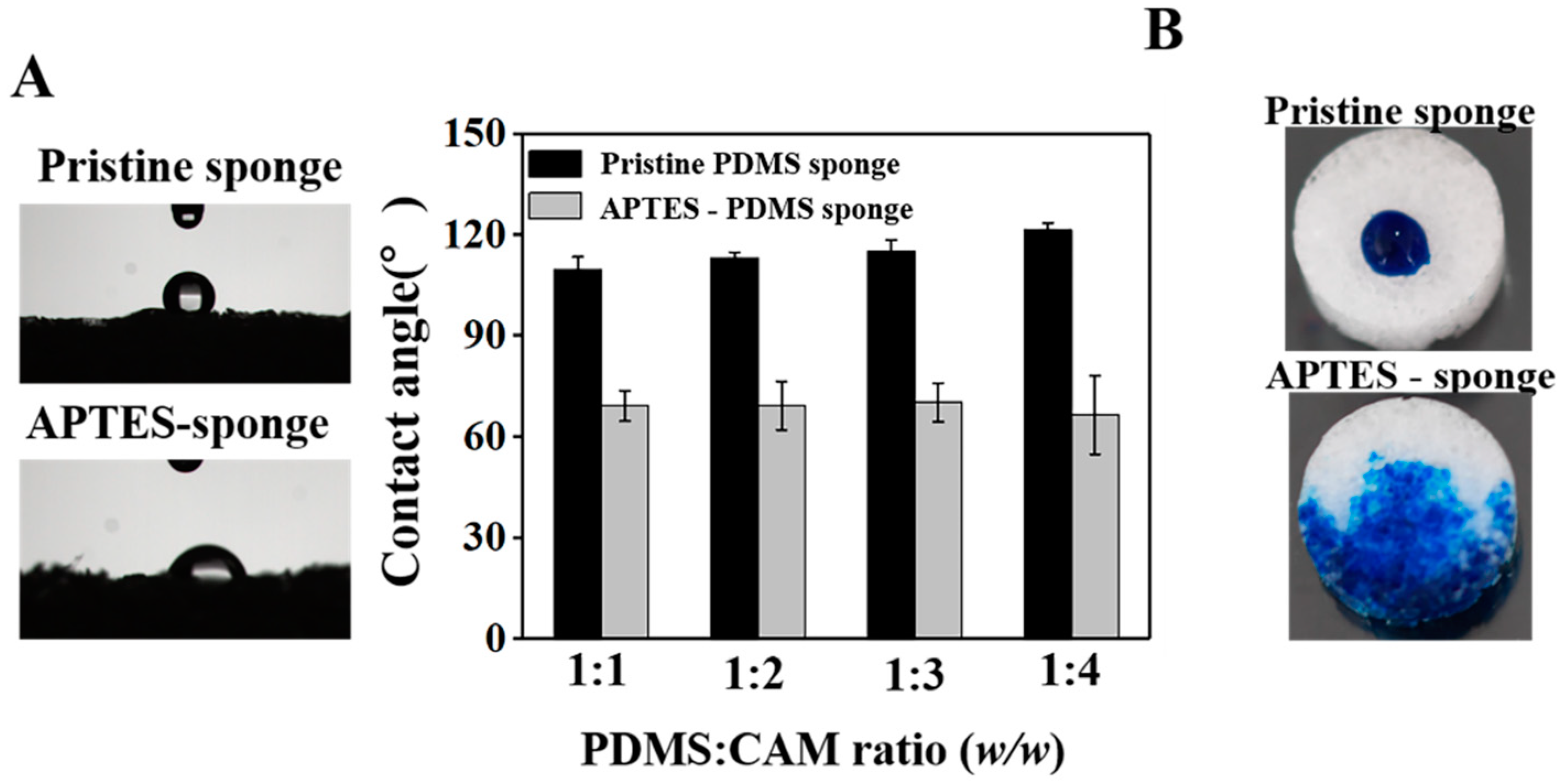
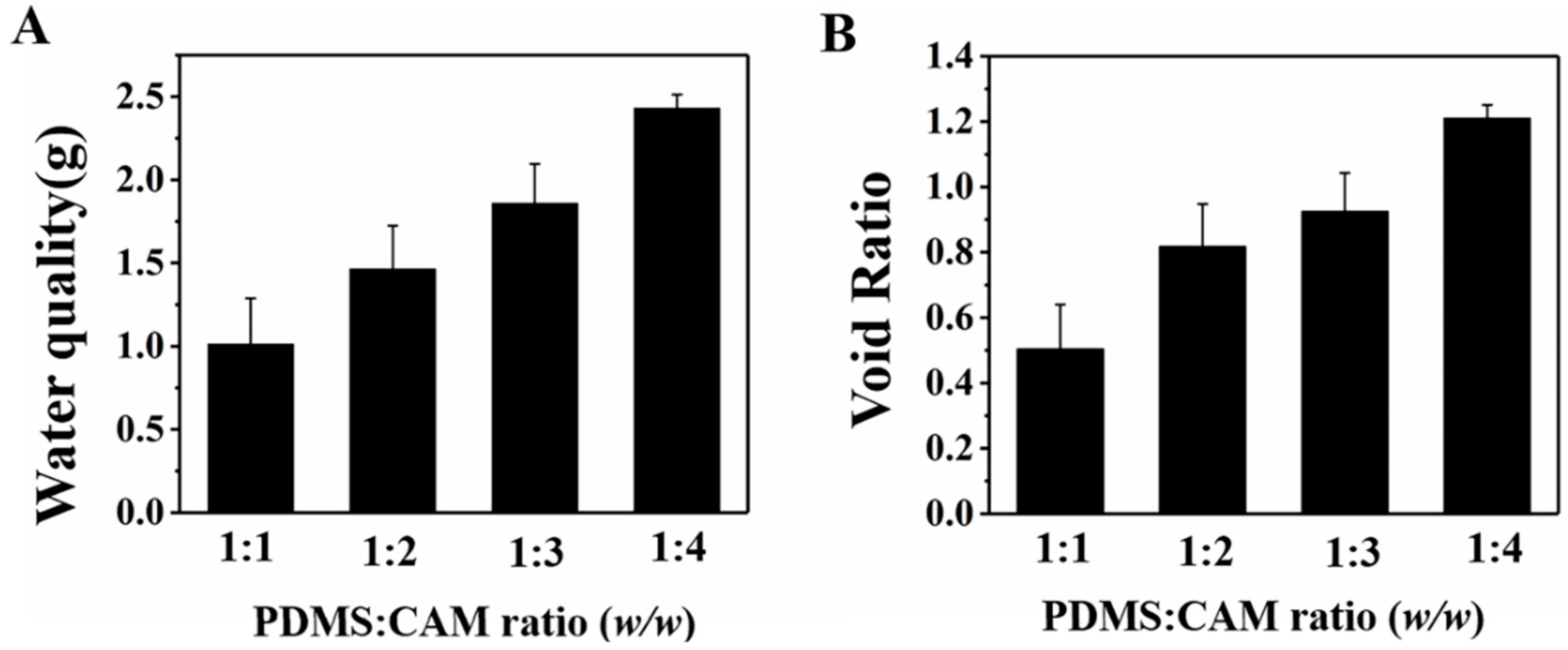
3.1. Improved Hydrophilicity to Ensure the Water Supplied for Plant Cultivation
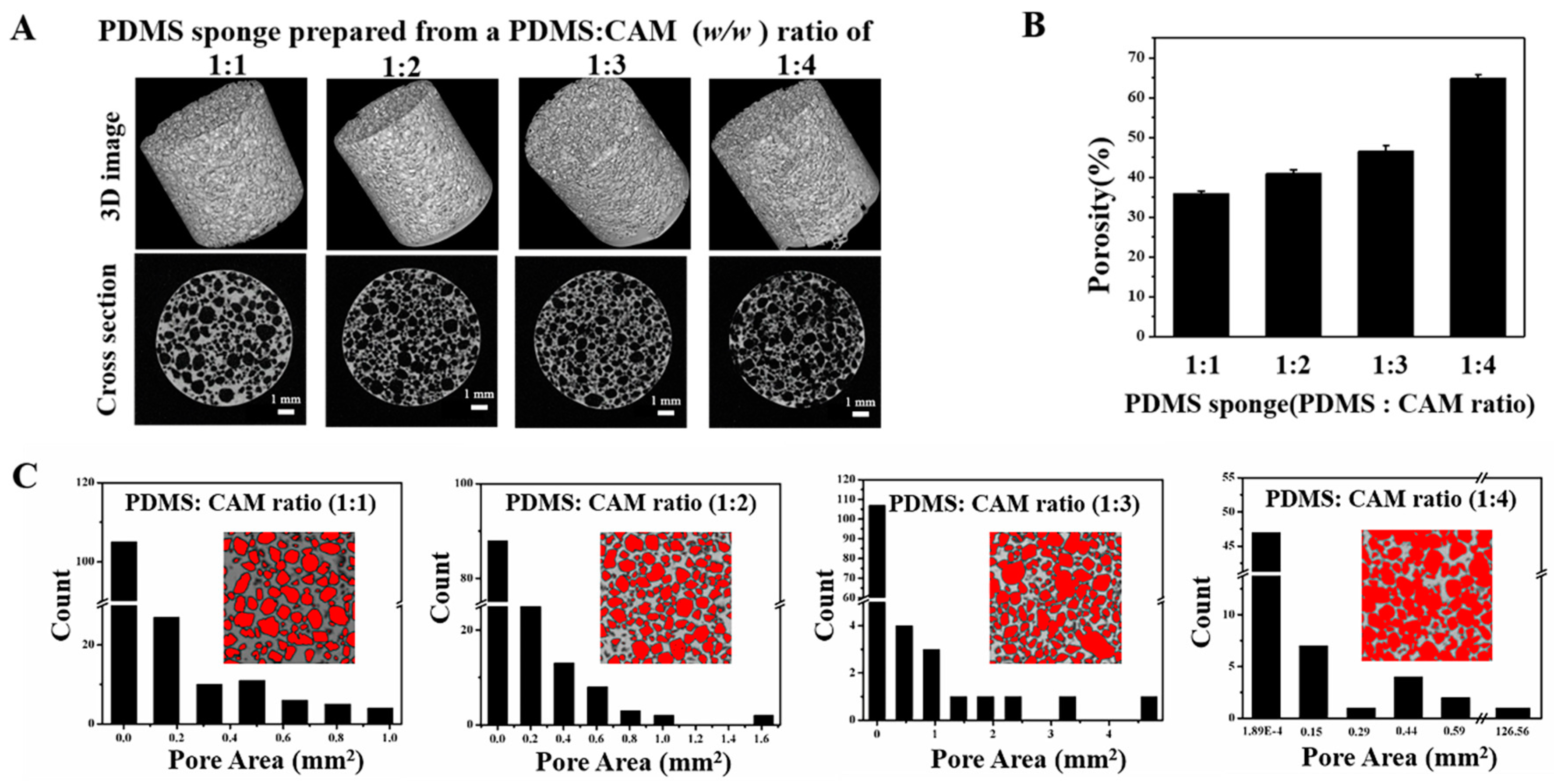
3.2. Porous PDMS Sponge to Mimic Cavities in the Soil
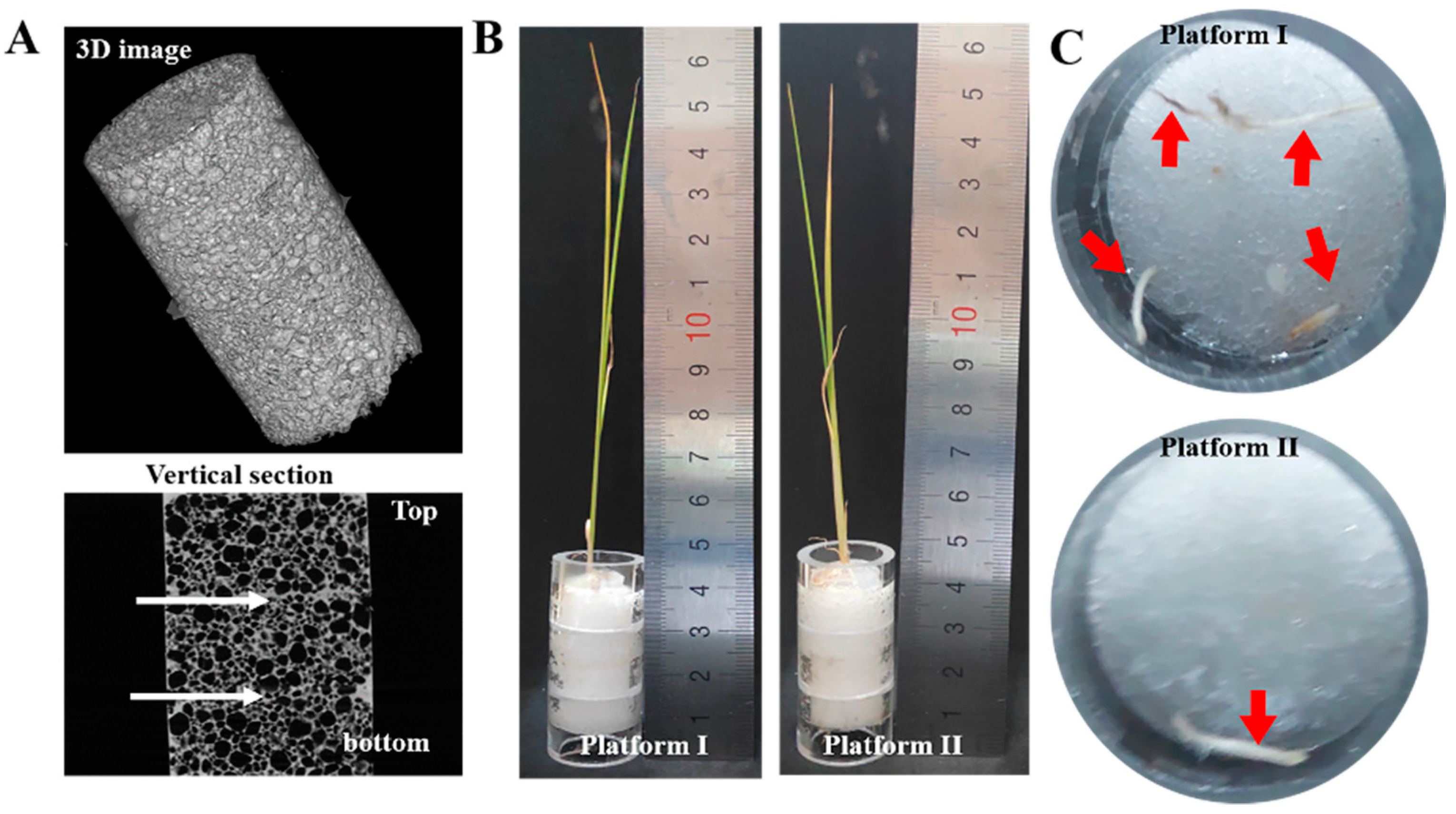
3.3. Plant Growth on Hydrophilic Porous PDMS Sponge
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, K. From plant to power. Nature 2009, 461, 710. [Google Scholar] [CrossRef] [PubMed]
- Malá, J.; Cvrčkova, H.; Máchová, P.; Dostál, J.; Šíma, P. Heavy metal accumulation by willow clones in short-time hydroponics. J. For. Sci. 2010, 56, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Callaway, E. Lab staple agar hit by seaweed shortage. Nature 2015, 528, 171–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeren, P.V.D.; Vleeschauwer, D.D.; Debergh, P. Determination of oxygen profiles in agar-based gelled in vitro plant tissue culture media. Plant Cell Tissue Org. Cult. 2001, 65, 239–245. [Google Scholar] [CrossRef]
- Xu, W.; Ding, G.; Yokawa, K.; Baluska, F.; Li, Q.-F.; Liu, Y.; Shi, W.; Liang, J.; Zhang, J. An improved agar-plate method for studying root growth and response of arabidopsis thaliana. Sci. Rep. 2013, 3, 1273. [Google Scholar] [CrossRef] [Green Version]
- EmmeT-Booth, J.P.; Forristal, P.D.; Fenton, O.; Ball, B.C.; Holden, N.M. A review of visual soil evaluation techniques for soil structure. Soil Use Manag. 2016, 32, 623–634. [Google Scholar] [CrossRef]
- Milde, G.A.; Dedecek, R.A.; Gava, J.L. Average soil particles diameter to predict some soil physical properties. Braz. J. For. Res. 2010, 57, 21–27. [Google Scholar]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, A.; Oggiano, N.; Maletta, M.; Rinaldi, M. Fertirrigation systems and substrates in soilless gerbera cultivation. Colt. Protette 2000, 98, 2291–2296. [Google Scholar]
- Dyśko, J.; Kowalczyk, W.; Kaniszewski, s. The influence of ph of nutrient solution on yield and nutritional status of tomato plants grown in soilless culture system. Vegetable Crops Res. Bull. 2009, 70, 59–69. [Google Scholar] [CrossRef]
- Dunlop, S.J.; Arbestain, M.C.; Bishop, P.; Wargent, J.J. Closing the loop: Use of biochar produced from tomato crop green waste as a substrate for soilless, hydroponic tomato production. Hortscience 2015, 50, 1572–1581. [Google Scholar] [CrossRef]
- Vainburg, V.M.; Lysenko, A.A.; Shtyagina, L.M.; Illarionova, E.L.; Chufarovskaya, T.I.; Sverdlova, N.I. Fibrous materials as artificial soil substrates. Fiber Chem. 2008, 40, 308–313. [Google Scholar] [CrossRef]
- Sterne, R.E.; McCarver, T.H. Formation of sporangia by Phytophthora cryptogea and P. parasitica in artificial and natural soils. Soil Biol. Biochem. 1980, 12, 441–442. [Google Scholar] [CrossRef]
- Hwang, G.; Lee, C.-H.; Ahn, I.-S.; Mhin, B.J. Analysis of the adhesion of pseudomonas putida ncib 9816-4 to a silica gel as a model soil using extended dlvo theory. J. Hazard. Mater. 2010, 179, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Patrick, H. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, N.; Choi, G.; Park, J.-K. Plant array chip for the germination and growth screening of arabidopsis thaliana. Lab Chip 2017, 17, 3071–3077. [Google Scholar] [CrossRef]
- Grossmann, G.; Guo, W.J.; Ehrhardt, D.W.; Frommer, W.B.; Meier, M. The rootchip: An integrated microfluidic chip for plant science. Plant Cell 2011, 23, 4234–4240. [Google Scholar] [CrossRef] [Green Version]
- Chai, H.; Chen, F.; Zhang, S.J.; Li, Y.D.; Yu, L. Multi-chamber petaloid root-growth microfluidic chip for the non-destructive study of the development and physiology of the fibrous root systems of oryza sativa. Lab Chip 2019, 19, 2383–2393. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in pdms surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Rooij, G.H.D. Methods of soil analysis. Catena 2004, 3, 722. [Google Scholar] [CrossRef]
- Krishna, R.; Siraj, P.; Sastry, C. Spectrophotometric determination of citric acid with metol and chromium (VI). Fresen J. Anal. Chem. 1980, 303, 411–417. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Zhang, X. Determination of organic acids in the root exudates of Cr-hyperaccumulator Leersia hexandra Swarts using high performance liquied chromatography. Chin. J. Chromatogr. 2018, 36, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Aulakh, M.; Wassmann, R.; Bueno, C.; Kreuzwieser, J.; Rennenberg, H. Characterization of root exudates at different growth stage of ten rice (Oryza sativa L.) cultivars. Plant. Biol. 2001, 3, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, P.; Das, S.; Adhya, T. Root exudates of rice cultivars affect rhizospheric phosphorus dynamics in soils with different phosphorus statuses. Commun. Soil Plant Anal. 2013, 44, 1643–1658. [Google Scholar] [CrossRef]
- Dishman, K.; Doolin, P.; Hoffman, J. Comparison of particle size of cracking catalyst determined by laser linght scattering and dry sieve methods. Ind. Eng. Chem Res. 1993, 32, 1457–1463. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Chai, H.; Song, Z.; Yu, L.; Fang, C. Hydrophilic Porous Polydimethysiloxane Sponge as a Novel 3D Matrix Mimicking Heterogeneous Pores in Soil for Plant Cultivation. Polymers 2020, 12, 140. https://doi.org/10.3390/polym12010140
Chen F, Chai H, Song Z, Yu L, Fang C. Hydrophilic Porous Polydimethysiloxane Sponge as a Novel 3D Matrix Mimicking Heterogeneous Pores in Soil for Plant Cultivation. Polymers. 2020; 12(1):140. https://doi.org/10.3390/polym12010140
Chicago/Turabian StyleChen, Feng, Huihui Chai, Zhaoxi Song, Ling Yu, and Can Fang. 2020. "Hydrophilic Porous Polydimethysiloxane Sponge as a Novel 3D Matrix Mimicking Heterogeneous Pores in Soil for Plant Cultivation" Polymers 12, no. 1: 140. https://doi.org/10.3390/polym12010140