Enhanced Poly(propylene carbonate) with Thermoplastic Networks: A Cross-Linking Role of Maleic Anhydride Oligomer in CO2/PO Copolymerization
Abstract
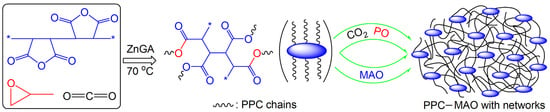
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Copolymerization Procedure
2.3. Characterization and Measurements
3. Results and Discussions
3.1. Synthesis
3.2. Thermal Properties
3.3. Mechanical Properties
3.4. Dimentional Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beckman, E.J. Making polymers from carbon dioxide. Science 1999, 283, 946–947. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coord. Chem. Rev. 2018, 372, 85–100. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150085. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-B.; Ren, W.-M.; Wu, G.-P. CO2 Copolymers from Epoxides: Catalyst Activity, Product Selectivity, and Stereochemistry Control. Acc. Chem. Res. 2012, 45, 1721–1735. [Google Scholar] [CrossRef] [PubMed]
- Sujith, S.; Min, J.K.; Seong, J.E.; Na, S.J.; Lee, B.Y. A Highly Active and Recyclable Catalytic System for CO2/Propylene Oxide Copolymerization. Angew. Chem. 2008, 120, 7416–7419. [Google Scholar]
- Luinstra, G.A. Poly(Propylene Carbonate), Old Copolymers of Propylene Oxide and Carbon Dioxide with New Interests: Catalysis and Material Properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Zhang, D.; Boopathi, S.K.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Metal-Free Alternating Copolymerization of CO2 with Epoxides: Fulfilling “Green” Synthesis and Activity. J. Am. Chem. Soc. 2016, 138, 11117–11120. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Borchardt, E. Material properties of poly(propylene carbonates). Adv. Polym. Sci. 2012, 245, 29–48. [Google Scholar]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T.; Sun, L.; Meng, Y. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Qin, Y.S.; Sheng, X.F.; Liu, S.J.; Ren, G.J.; Wang, X.H.; Wang, F.S. Recent advances in carbon dioxide based copolymers. J. CO2 Util. 2015, 11, 3–9. [Google Scholar] [CrossRef]
- Nakano, K.; Hashimoto, S.; Nakamura, M.; Kamada, T.; Nozaki, K. Stereocomplex of Poly(propylene carbonate): Synthesis of Stereogradient Poly(propylene carbonate) by Regio- and Enantioselective Copolymerization of Propylene Oxide with Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, G.-P.; Ren, W.-M.; Wang, Y.-M.; Rao, D.-Y.; Lu, X.-B.; Wu, G.; Ren, W.; Wang, Y.; Rao, D.; et al. Asymmetric, regio- and stereo-selective alternating copolymerization of CO2 and propylene oxide catalyzed by chiral chromium Salan complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6102–6113. [Google Scholar] [CrossRef]
- Gao, J.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Bai, H.; Fu, Q. A promising alternative to conventional polyethylene with poly(propylene carbonate) reinforced by graphene oxide nanosheets. J. Mater. Chem. 2011, 21, 17627–17630. [Google Scholar] [CrossRef]
- Chen, L.J.; Qin, Y.S.; Wang, X.H.; Li, Y.S.; Zhao, X.J.; Wang, F.S. Toughening of poly(propylene carbonate) by hyperbranched poly(ester-amide) via hydrogen bonding interaction. Polym. Int. 2011, 60, 1697–1704. [Google Scholar] [CrossRef]
- Song, P.F.; Wang, S.J.; Xiao, M.; Du, F.G.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Cross-linkable and thermally stable aliphatic polycarbonates derived from CO2, propylene oxide and maleic anhydride. J. Polym. Res. 2009, 16, 91–97. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, X.; Zhao, X.; Li, J.; Wang, F. Crosslinkable poly(propylene carbonate): High-yield synthesis and performance improvement. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5329–5336. [Google Scholar] [CrossRef]
- Wang, X.L.; Meng, Y.Z.; Li, R.K.Y. Crosslinking of poly(propylene carbonate) by peroxide crosslinking agent dicumyl peroxide (DCP). Acta Sci. Nat. Univ. Sunyatseni 2007, 46, 4–6. [Google Scholar]
- Hao, Y.P.; Ge, H.H.; Han, L.J.; Liang, H.Y.; Zhang, H.L.; Dong, L.S. Thermal, mechanical, and rheological properties of poly(propylene carbonate) cross-linked with polyaryl polymethylene isocyanate. Polym. Bull. 2013, 70, 1991–2003. [Google Scholar] [CrossRef]
- Qin, Y.S.; Ma, Q.W.; Wang, X.H.; Sun, J.Z.; Zhao, X.J.; Wang, F.S. Electron-beam irradiation on poly(propylene carbonate) in the presence of polyfunctional monomers. Polym. Degrad. Stab. 2007, 92, 1942–1947. [Google Scholar] [CrossRef]
- Xia, L.; Chen, L.B. Silicone modified poly(propylene carbonate). Polym. Mater. Sci. Eng. 2003, 19, 202–204. [Google Scholar]
- Darensbourg, D.J.; Wang, Y.Y. Terpolymerization of propylene oxide and vinyl oxides with CO2: Copolymer cross-linking and surface modification via thiol-ene click chemistry. Polym. Chem. 2015, 6, 1768–1776. [Google Scholar] [CrossRef]
- Song, P.F.; Mao, X.D.; Liu, X.J.; Ji, X.Q.; Zhang, X.F.; Wang, R.M. Study on synthesis and properties of terpolymers derived from carbon dioxide, propylene oxide and γ-glycidyloxypropyltrimethoxysilane. Mater. Rev. 2013, 27, 82–84, 100. [Google Scholar]
- Cyriac, A.; Lee, B.Y.; Lee, S.H. Connection of polymer chains using diepoxide in CO2/propylene oxide copolymerizations. Polym. Chem. 2011, 2, 950. [Google Scholar] [CrossRef]
- Okada, A.; Kikuchi, S.; Yamada, T. Alternating Copolymerization of Propylene Oxide/Alkylene Oxide and Carbon Dioxide: Tuning Thermal Properties of Polycarbonates. Chem. Lett. 2011, 40, 209–211. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, M.; He, H.; Wang, S.; Han, D.; Meng, Y. Copolymerization of propylene oxide and carbon dioxide in the presence of diphenylmethane diisoyanate. J. Polym. Res. 2011, 18, 1479–1486. [Google Scholar] [CrossRef]
- Gao, L.J.; Feng, J.Y. A one-step strategy for thermally and mechanically reinforced pseudo-interpenetrating poly(propylene carbonate) networks by terpolymerization of CO2, propylene oxide and pyromellitic dianhydride. J. Mater. Chem. A 2013, 1, 3556–3560. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Gao, L.-J.; Chen, B.; Wu, X.-J.; Luo, Q.-L.; Wu, C.-Y.; Zheng, C.-X.; Lin, L.-Z.; Deng, S.-L.; Huang, X.-M. A One-step Strategy for Reinforced Poly(propylene carbonate) with Partial Crosslinking via Terpolymerization of CO2 and Propylene Oxide Using Triglycidyl Isocyanurate. Chem. Lett. 2013, 42, 714–716. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Feng, J.; Huang, X.; Guo, X.; Chen, J.; Xiao, Z.; Liang, X.; Gao, L. Enhanced Poly(Propylene Carbonate) with Thermoplastic Networks: A One-Pot Synthesis from Carbon Dioxide, Propylene Oxide, and a Carboxylic Dianhydride. Polymers 2018, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Song, P.F.; Mao, X.D.; Zhang, X.F.; Zhu, X.G.; Wang, R.M. A one-step strategy for cross-linkable aliphatic polycarbonates with high degradability derived from CO2, propylene oxide and itaconic anhydride. RSC Adv. 2014, 4, 15602–15605. [Google Scholar] [CrossRef]
- Gao, L.J.; Chen, X.G.; Liang, X.J.; Guo, X.Z.; Huang, X.L.; Chen, C.F.; Wan, X.D.; Deng, R.Y.; Wu, Q.F.; Wang, L.Y.; et al. A novel one-pot synthesis of poly(propylene carbonate) containing cross-linked networks by copolymerization of carbon dioxide, propylene oxide, maleic anhydride, and furfuryl glycidyl ether. Polymers 2019, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, X.; Zhao, X.; Li, J.; Wang, F. Double propagation based on diepoxide, a facile route to high molecular weight poly(propylene carbonate). Polymer 2006, 47, 7368–7373. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Zhang, H.; Ding, H.; Liu, B.; Wang, X. One-pot synthesis and postpolymerization functionalization of cyclic carbonate/epoxide-difunctional polycarbonates prepared by regioselective diepoxide/CO2 copolymerization. Polym. Chem. 2016, 7, 4453–4457. [Google Scholar] [CrossRef]
- Cyriac, A.; Jeon, J.Y.; Lee, B.Y.; Lee, S.H. Preparation of thermoplastic polyurethanes using in situ generated poly(propylene carbonate)-diols. Polym. Chem. 2012, 3, 1215. [Google Scholar]
- DiCiccio, A.M.; Coates, G.W. Ring-Opening Copolymerization of Maleic Anhydride with Epoxides: A Chain-Growth Approach to Unsaturated Polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef]
- Bao, Y.-Y.; Liu, Y.; Ren, W.-M.; Lu, X.-B.; Liu, J. Binuclear chromium–salan complex catalyzed alternating copolymerization of epoxides and cyclic anhydrides. Polym. Chem. 2013, 4, 1439–1444. [Google Scholar]
- Van Zee, N.J.; Coates, G.W. Alternating Copolymerization of Propylene Oxide with Biorenewable Terpene-Based Cyclic Anhydrides: A Sustainable Route to Aliphatic Polyesters with High Glass Transition Temperatures. Angew. Chem. Int. Ed. 2015, 54, 2665–2668. [Google Scholar] [CrossRef]
- Hua, Z.; Qi, G.; Chen, S. Ring-opening copolymerization of maleic anhydride with propylene oxide by double-metal cyanide. J. Appl. Polym. Sci. 2004, 93, 1788–1792. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Wang, S.; Xia, L.; Hang, D.; Cui, G.; Meng, Y. Mechanism studies of terpolymerization of phthalic anhydride, propylene epoxide, and carbon dioxide catalyzed by ZnGA. RSC Adv. 2014, 4, 9503–9508. [Google Scholar] [CrossRef]
- McCrone, J.D.; Guo, H.; Meador, M.A.B.; McCorkle, L.S.; Scheiman, D.A.; Wilkewitz, B. Poly(maleic anhydride) cross-linked polyimide aerogels: Synthesis and properties. RSC Adv. 2016, 6, 26055–26065. [Google Scholar]
- Kascholke, C.; Loth, T.; Kohn-Polster, C.; Möller, S.; Bellstedt, P.; Schulz-Siegmund, M.; Schnabelrauch, M.; Hacker, M.C. Dual-Functional Hydrazide-Reactive and Anhydride-Containing Oligomeric Hydrogel Building Blocks. Biomacromolecules 2017, 18, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Regel, W.; Schneider, C. Poly(maleic anhydride)—Synthesis and proof of structure. Die Makromol. Chem. 1981, 182, 237–242. [Google Scholar] [CrossRef]
- Hussain, K.F.; Al-Roomi, Y.M. Homo-oligomerization of maleic anhydride in nonpolar solvents: A kinetic study of deviations from nonlinear behavior. J. Appl. Polym. Sci. 2006, 102, 3404–3412. [Google Scholar]
- Tang, L.; Xiao, M.; Xu, Y.; Wang, S.; Meng, Y. Zinc adipate/tertiary amine catalytic system: Efficient synthesis of high molecular weight poly(propylene carbonate). J. Polym. Res. 2013, 20, 190. [Google Scholar] [CrossRef]
- Li, X.; Meng, Y.; Zhu, Q.; Tjong, S. Thermal decomposition characteristics of poly(propylene carbonate) using TG/IR and Py-GC/MS techniques. Polym. Degrad. Stab. 2003, 81, 157–165. [Google Scholar] [CrossRef]
- Peng, S.; An, Y.; Chen, C.; Fei, B.; Zhuang, Y.; Dong, L. Thermal degradation kinetics of uncapped and end-capped poly(propylene carbonate). Polym. Degrad. Stab. 2003, 80, 141–147. [Google Scholar] [CrossRef]
- An, J.J.; Ke, Y.C.; Cao, X.Y.; Ma, Y.M.; Wang, F.S. A novel method to improve the thermal stability of poly (propylene carbonate). Polym. Chem. 2014, 5, 4245–4250. [Google Scholar] [CrossRef]
- Song, P.F.; Xiao, M.; Du, F.G.; Wang, S.J.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z.; Wang, S. Synthesis and properties of aliphatic polycarbonates derived from carbon dioxide, propylene oxide and maleic anhydride. J. Appl. Polym. Sci. 2008, 109, 4121–4129. [Google Scholar] [CrossRef]





| Sample | MAO Ratio of PO (wt%) | Yield (g Polymer/g ZnGA) | Gel (%) | Selectivity a (% PPC) | Carbonate Linkages a (%) |
|---|---|---|---|---|---|
| PPC | 0 | 26 | 0 | 96.4 | 98.2 |
| PPC–MAO0.625 | 0.625 | 53 | 14.2 ± 1.2 | 96.7 | 96.2 |
| PPC–MAO1.25 | 1.25 | 58 | 17.5 ± 1.3 | 96.6 | 95.5 |
| PPC–MAO2.5 | 2.5 | 64 | 21.4 ± 1.6 | 98.3 | 94.4 |
| PPC–MAO3.75 | 3.75 | 67 | 27.3 ± 2.1 | 98.3 | 94.6 |
| PPC–MAO5 | 5 | 72 | 33.6 ± 2.3 | 97.9 | 94.1 |
| Sample | Td,−5% (°C) | Td,max (°C) | Tg (°C) |
|---|---|---|---|
| PPC | 215.0 | 228.7, 256.0 | 35.5 |
| PPC–MAO0.625 | 283.4 | 297.9 | 31.8 |
| PPC–MAO1.25 | 287.1 | 300.3 | 34.4 |
| PPC–MAO2.5 | 287.3 | 302.5 | 36.2 |
| PPC–MAO3.75 | 287.7 | 302.5 | 33.7 |
| PPC–MAO5 | 289.8 | 308.8 | 32.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Huang, M.; Wu, Q.; Wan, X.; Chen, X.; Wei, X.; Yang, W.; Deng, R.; Wang, L.; Feng, J. Enhanced Poly(propylene carbonate) with Thermoplastic Networks: A Cross-Linking Role of Maleic Anhydride Oligomer in CO2/PO Copolymerization. Polymers 2019, 11, 1467. https://doi.org/10.3390/polym11091467
Gao L, Huang M, Wu Q, Wan X, Chen X, Wei X, Yang W, Deng R, Wang L, Feng J. Enhanced Poly(propylene carbonate) with Thermoplastic Networks: A Cross-Linking Role of Maleic Anhydride Oligomer in CO2/PO Copolymerization. Polymers. 2019; 11(9):1467. https://doi.org/10.3390/polym11091467
Chicago/Turabian StyleGao, Lijun, Meiying Huang, Qifeng Wu, Xiaodan Wan, Xiaodi Chen, Xinxin Wei, Wenjing Yang, Rule Deng, Lingyun Wang, and Jiuying Feng. 2019. "Enhanced Poly(propylene carbonate) with Thermoplastic Networks: A Cross-Linking Role of Maleic Anhydride Oligomer in CO2/PO Copolymerization" Polymers 11, no. 9: 1467. https://doi.org/10.3390/polym11091467