1. Introduction
The PET usage as a packaging material increases year by year, and therefore the PET bottle waste also expands rapidly due to the fact that the life of a plastic bottle is brief [
1,
2]. The recycling of the PET waste is an important environmental question and the answer could be the upgrading recycling of flakes to technological plastics even if the longer lifetime is required by the potential application fields. It is claimed that morphological and mechanical properties of polyesters are just slightly decreasing with recycling when the optimal technology is applied [
3,
4], however the degradation which is characterized by the value of intrinsic viscosity (IV) could increase the crystallinity and the modulus of the material and decrease the impact strength [
5,
6]. Nevertheless, there is always an opportunity to improve these properties, e.g., by using fiber reinforcement, blending with other polymers or adding some extra additives [
7,
8,
9,
10]. Quality improvement could be achieved in other aspects of the polymer as well, such as by increasing its fire retardancy [
11,
12,
13] and thus open the possibility for new applications, such as in electrical and electronic products.
Nowadays, it is a global industrial problem that an increasing number of common fire retardants that proved to be effective even in smaller doses, such as systems containing halogen, are now forbidden or under a process of restriction. The presently used alternative solutions, such as metal-oxides, metal-hydroxides, phosphorous compounds etc. are usually expensive, and need to be used in larger quantities that make them even more costly. Moreover, they often cause deterioration of the mechanical properties [
14,
15,
16,
17,
18]. The researchers strive to develop fire retardants that can satisfy the standards but do not raise the price of the products significantly and do not decrease the mechanical properties of the polymer, in ideal situations they even improve it [
19,
20].
In the case of halogen-free aluminum-alkylphosphinate (AlPi), the dominant flame retardant mechanism is through the release of phosphinate compounds that inhibit the chemistry of the gas-phase combustion and the increase of the carbonaceous residue (char) production that invoke a thermal barrier effect [
21]. Besides, it changes the melt viscosity of the matrix polymer and therefore increases the dripping behavior [
22,
23].
The flame retardant effect of nanostructured materials is widely investigated. Adding carbon nanotubes (CNT) [
24,
25], montmorillonite (MMT) [
26,
27], boehmite [
28] or sepiolite [
29] the flammability of polyesters can be reduced, although using these substances alone, high level of flame retardancy (e.g., V0 classification according to UL-94 standard) cannot be achieved. The use of MMT as a synergic additive to AlPi for designing polyesters with better flame retardant properties has already been studied by researchers, however, there are some contradictory results in the literature about the required quantities and the necessity and nature of the surface treatments.
Ye et al. [
30] analyzed poly(lactic acid) (PLA) with AlPi and natural MMT modified with methyl-tallow-bis(2-hydroxyethyl) ammonium. Authors concluded that the dominant reaction was the char formation and melt dripping disappeared when either FR or oMMT was added to the matrix. V0 classification according to the UL-94 standard was only achieved when 17% AlPi and 3% oMMT were used together.
Kim et al. [
31] investigated the effects of MMT modified with phosphonium salt of dodecyltriphenyl in in situ polymerized poly(butylene terephthalate) (PBT) matrix. According to the authors, the initial decomposition temperature increased slightly when 1–2% of modified MMT was used.
Ramani et al. [
23] concluded that there is a synergetic effect when 2.5% quaternary ammonium salt modified MMT (oMMT) and 15.5% AlPi was added to glass fiber reinforced PBT (GF-PBT). Due to the addition of 2.5% oMMT to the flame retardant composite, the limiting oxygen index (LOI) value increased from 31.5 to 35.5%. In this study, it was established that the presence of AlPi led to char formation while adding oMMT led to the formation of inorganic deposits that increased the viscosity of the GF-PBT. Based on cone-calorimetry using different external heat fluxes, it was shown that AlPi flame retardant with oMMT was more resistant to ignition than the rest of the materials at the lower heat flux (22.5 kW/m
2). This was explained by the water content in the crystal lattice, which induces the hydrolytical decomposition of AlPi to produce phosphorus containing radicals. This could increase the
TTI through radical scavenging mechanisms. During decomposition of MMT, water and carbon dioxide gases were produced which diluted the decomposing olefinic compounds emerging from the disruption of the polyester matrix. Based on the authors experiment at a higher external heat flux (30–90 kW/m
2) the crystalline water escapes before reacting with AlPi and at the same time the phosphorous radical species also escape the flame front. Hence the
TTI is shorter when compared with PBT containing only flame retardant.
Louisy et al. [
32] tested similar FR compositions in GF-PBT matrix. 20% AlPi and 18% AlPi + 2% MMT modified with quaternary ammonium salt were added. Composite with oMMT content showed slightly lower LOI value compared to the composite containing only AlPi (39% to 40%).
Ge et al. [
33] investigated the effect of oMMT (organically modified by octadecyltrimethyl-ammonium chloride) on PET–2-carboxyethyl(phenylphosphinic) acid (PET-
co-HPPPA) copolymer. HPPPA content was 5% and the ratio of oMMT was varied between 1–3%. LOI index shifted from 31.5% to 34% by adding 1% of oMMT and it did not change after any further increase of oMMT content. UL-94 results show V2 rating at 0 and 1%, and V0 at 2 and 3% oMMT content.
Habibi et al. [
34] prepared PET-oMMT nanocomposites with 0, 3 and 5% oMMT content. Based on their cone-calorimeter tests it was found that flame retardant properties of nanocomposites improved with increasing clay content. The nanocomposite containing 5% oMMT showed adequate flame retardancy and dripping resistance. Besides, decreasing LOI values of the PET/oMMT composites ware measured with increasing oMMT content.
The reason for developing PET-MMT systems is primarily not only the flame retardancy of the material but the ability to improve the gas barrier and mechanical properties [
35,
36,
37,
38,
39]. The role of surface modification in the development of properties is an intensively researched area.
Pegoretti et al. [
40] used montmorillonites without modification and ion-exchanged MMT modified by quaternary ammonium salts and added to regranulated PET flakes. By analyzing the nanocomposites’ mechanical and morphological properties they established that the composites containing modified MMT were able to form an intercalated structure. However, only a small interlayer space shift was observed when unmodified MMT was added to the PET matrix, indicating weak intercalation.
Wang et al. [
41] researched original PET nanocomposites with added organo-modified MMT. The interlayer spacing of the organo-modified MMT increased which was explained by intercalation. By analyzing the mechanical properties, they found that by adding 1 wt % MMT to the system the yield stress and flexural strength improved, but when 3 or 5 wt % were added the mechanical properties deteriorated. These findings can be explained by the increasing quantity of the filler, which resulted in the decrease of dispersion and lower intercalation degree. The oMMT increased the heat deflection temperature (HDT) of the PET as a function of increasing filler content, however the impact strength decreased, which was explained by the decreasing moving ability of the molecules.
Kracalik et al. [
42] prepared recycled PET (rPET) composites using 5% clay in a twin-screw extruder and then compared the dispersion of the different types of MMTs. They evinced that by raising the polarity of the surface better delamination can be achieved, and by mixing with the polar PET intercalation is also possible. Decomposition of alkylammonium ethers of the organo-modifier influenced the PET degradation during the processing.
Zare summarized in his review article [
13] the benefits of using MMT in recycled polymers. The author showed that substantial increase of the modulus can be achieved by high MMT content, although the optimum strength and stiffness is around 2% MMT content, due to the fact that the mobility of the molecular chains is influenced by the nanofiller.
Vassiliou et al. [
43] prepared organo-modified MMT nanocomposites in in situ polymerization of PET. With these well dispersed nanoparticles substantial improvement of the strength of the composites was achieved.
In summary, there are contradictory results in the literature regarding the dispersibility of neat and organo-modified MMTs in polyester matrix materials, furthermore the mechanical properties of the nanocomposites are barely studied, especially when flame retardant compositions are investigated (
Table 1).
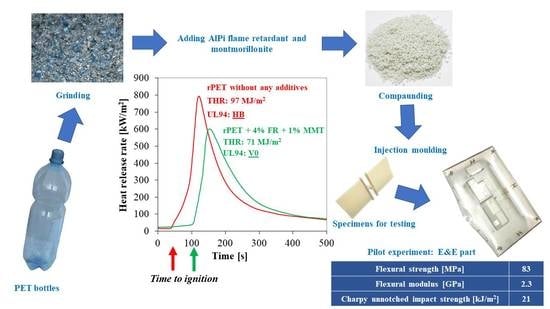
The aim of this study is the upgrading recycling of rPET by preparing flame retarded nanocomposites accompanied with adequate flammability and mechanical properties at the same time. V0 rating according to the UL-94 standard with reduced FR content was intended to be reached by optimizing the type and ratio of nanoclay type synergist. From the developed recycled material prototype of an electrical product (TV cover) was manufactured by injection molding and comprehensively characterized to demonstrate the feasibility of preparation of durable products from recycled raw materials.
2. Materials and Methods
rPET flakes (Jász-Plasztik Kft, Jászberény Hungary), originating from collected, washed and sorted post-consumer PET bottles, with an intrinsic viscosity (IV) value of 0.70 dL/g was used as matrix material. The average PE and PVC content of the rPET flakes was measured to be 25 and 20 ppm, respectively.
Exolit OP 1240 (Clariant, Muttenz, Switzerland) aluminum-tris-(diethylphosphinate) with a phosphorus content of 23.3–24.0% was used as flame retardant (FR) additive.
Cloisite 116 (Byk, Wesel, Germany) natural montmorillonite (MMT) and Cloisite 5 (Byk, Germany) natural montmorillonite modified with bis(hydrogenated tallow alkyl)dimethyl salt (oMMT) were used as nanofillers.
The rPET flakes were dried for 4 h at 150 °C, and then mixed together with the additives. LT 26-44 (Labtech Engineering, Samut Prakan, Thailand) twin screw extruder was used for the mixing with a melt temperature of 265 °C. The produced regranulate was dried for another 4 h at 150 °C, then 80 mm × 80 mm × 2 mm specimens were prepared by Allrounder Advance 370S 700-290 (Arburg, Lossburg, Germany) injection molding machine, with the following parameters: Melt temperature: 270 °C, maximum injection pressure: 900 bar, mold temperature: 60 °C.
Table 2 shows the composition of the test samples prepared for the optimization of the composition of the recycled product.
The interlayer spacing was measured in MMT and oMMT with wide-angle X-ray diffraction (WAXD). WAXD analysis was performed by PW 3710 (Philips, Amsterdam, the Nederland) based PW 1050 Bragg-Brentanopara focusing goniometer using CuKα radiation (λ = 0.15418 nm).
Thermogravimetric analysis (TGA) measurements were carried out on the used additives and prepared nanocomposites using a Labsys Evo (Setaram, Caluire-et-Cuire, France) instrument with a heating rate of 20 °C/min under nitrogen gas flow, covering a temperature range of 50–800 °C. About 6–8 mg of sample was used in each test.
SEM micrographs were obtained from the cryogenic fracture surfaces of the nanocomposites using EVO MA 10 instrument (Zeiss, Oberkochen, Germany) with an accelerating voltage of 30 kV. The samples were coated with 32 nm gold layer before examination in order to prevent charge build-up on the surface. The dispersion of the additives was investigated via energy dispersive X-ray spectrometry (EDS) using an Octane Pro type (AMATEX EDAX, Mahwah, NJ, USA) apparatus. In this case the thickness of the gold coating was 5 nm. Element mapping was carried out with an accelerating voltage of 15 keV and an amplification of 500×.
Mass loss type cone calorimeter tests were carried out by an instrument delivered by Fire Testing Technology Ltd. (East Grinstead, UK) based on the ISO 5660-1 standard method. 2 stacked pieces of injection molded specimens with dimensions of 80 mm × 80 mm × 2 mm were exposed to a constant heat flux of 50 kW/m
2 and ignited. Heat release values and mass reduction were continuously recorded during burning. The average effective heat of combustion (
AEHC) [MJ/kg] was calculated according to Equation (1), where
HRR [kW/m
2] is the heat release rate per unit exposed area,
Δt is the sampling time interval (in this case 1 s),
TTI is time to ignition,
EOF is time to end of flame and
m [kg/m
2] is the mass of specimen per unit exposed area. The fire performance index (
FPI) [sm
2/kW], a useful parameter that can be calculated as the ratio between the time to ignition (
TTI) [s] and the peak of heat release rate (
HRRmax) [kW/m
2], was calculated according to Equation (2).
The
FPI value gives important information about the degree of fire hazard [
44,
45].
The flame retardant performance of the prepared samples was characterized by limiting oxygen index (LOI) measurements according to the ASTM D 2863 standard. The LOI value expresses the lowest oxygen to nitrogen ratio where specimen combustion is still self-supporting.
Standard UL-94 tests were performed in a UL-94 chamber (Wazau, Berlin, Germany) with methane gas. Specimen thickness was 2 mm. UL-94 classification is used to determine dripping and flame spreading rates. First, horizontal burning tests were carried out. As long as the burning rate did not exceed 75 mm/min over a 75 mm span, the specimen got HB classification. If the burning stopped before it reached the 25 mm mark on the specimen, then the vertical burning test was carried out as well.
Three-point-bending tests were carried out using Z020 type (Zwick, Ulm, Germany) universal testing instrument (Zwick, Ulm, Germany) at room temperature. The test speed was 5 mm/min, support span was 64 mm.
Impact tests were carried out by Resil Impactor Junior (Ceast, Pianezza, Italy), using notched specimens. The measurements were performed at room temperature with a pendulum of 2 J and with a velocity of 2.9 m/s.
The morphological characteristics of RPET injection molded specimens were determined with a TA Q2000 type (TA Instruments, USA) DSC device at a heating rate of 10 °C/min under 25 mL/min nitrogen gas flow, covering the temperature range of 20 and 290 °C (one heating cycle). The weight of the examined samples was between 6 and 8 mg. Crystalline fraction (
CRF) was calculated by equation (Equation (3)):
where
CRF is crystalline fraction in the sample [%],
Δhm is the specific enthalpy of melting [J/g],
Δhcc is the specific enthalpy of cold crystallization [J/g],
Δhm0 is the specific melting enthalpy of 100% crystalline PET (140.1 J/g) and
α is the ratio of additives [
46].
The intrinsic viscosity (IV) of the PET material and the specimens was determined using a computer controlled PSL Rheotek automatic solution viscometer equipped with an optical sensor. Phenol-tetrachloroethane mixture in the ratio of 60/40 was applied as a solvent—the concentration was 0.5 g/dL, and examination temperature was 30 °C.
4. Conclusions
In this work, the type (untreated or organomodified) and amount (1 to 3%) of montmorillonite type nanosynergists were investigated on the flammability and mechanical performance of recycled PET flame retarded with aluminum-alkylphosphinate with the aim to find the best flame retardant composition for production of a technical product from recycled PET.
The cone calorimetric measurements showed that by combining the FR with untreated MMT the heat release rate of the rPET composites was noticeably moderated. Due to their synergetic effect, when 4% metal-phosphinate and 1 or 3 % MMT were used, the ignition time significantly increased. Based on HRRmax values better results were obtained with 1% nanoclay content than with 3% which can be explained by the difference in the dispersion. This assumption was confirmed by SEM and EDS measurements. By using oMMT the HRR maximum was only slightly moderated, and the TTI did not vary noticeably, either.
The flexural strength of the rPET composites decreased by using FR, however the extent of the decrease did not reach 10%. This value is among the best results that have been achieved nowadays. Both of the layered silicates resulted in an increase of the flexural modulus, accompanied by a decrease of the impact strength. A noticeable increase of the flexural strength was achieved with MMT addition, however by the addition of oMMT similar quality improvement could not be noticed. The distinct effect of the two kinds of nanofillers on the properties of the rPET composites can be traced back to the different degree of dispersion.
It was concluded that the combined application of AlPi and untreated MMT has several advantages; V0 rating according to the UL-94 standard is achievable with as low as 5% of additives (4% AlPi + 1% MMT) besides reaching an LOI value of 29%. Furthermore, improvement in flexural strength and modulus can be achieved without compromising the impact resistance of the flame retarded rPET composites.
The high-temperature processing and the additives caused degradation of the rPET macromolecules, which was traced by measuring the IV values by every processing steps. The reduced IV values are associated with reduced impact strength.
It was demonstrated that the developed recycled material, upgraded with flame retardancy and nanoclay type reinforcement, has comparable flame retardant performance and flexural properties as the polymers (PC/ABS, HIPS) that are currently widely applied in electrical parts. However, due to the unavoidable hydrolytic degradation of the macromolecules during reprocessing, the insufficient impact strength of the recycled material needs to be improved when considering its application in the electrical industry. Nevertheless, manufacturing of a television part was successfully accomplished by injection moulding of the rPET based material.