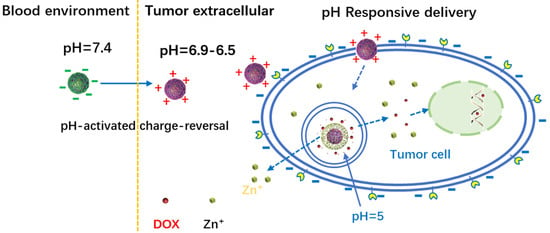
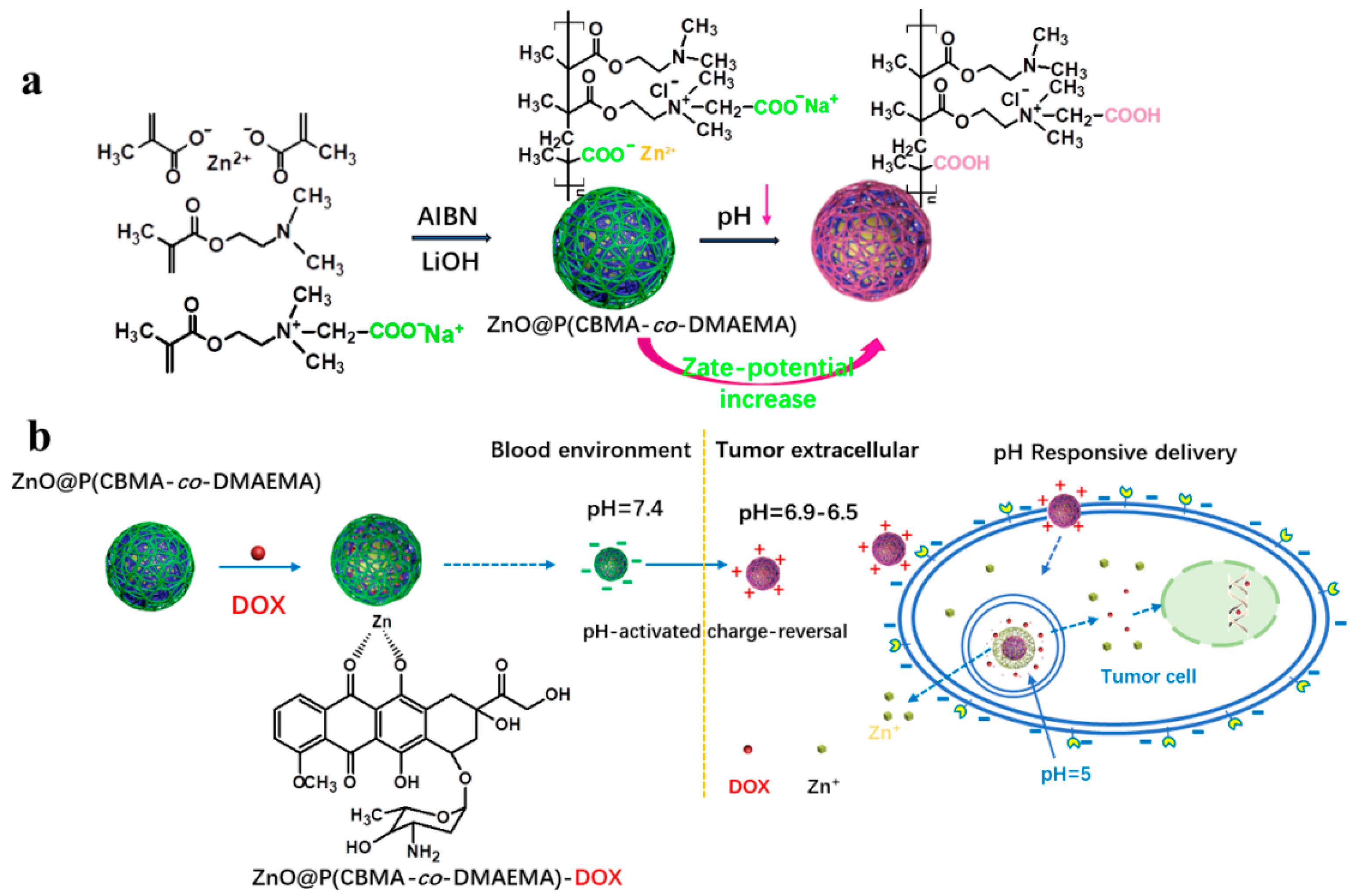
ZnO Quantum Dots Modified by pH-Activated Charge-Reversal Polymer for Tumor Targeted Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zinc Dimethacrylate
2.3. Preparation of ZnO@P(CBMA-co-DMAEMA) Hybrid Nanoparticles with Different CBMA/DMAEMA Ratios
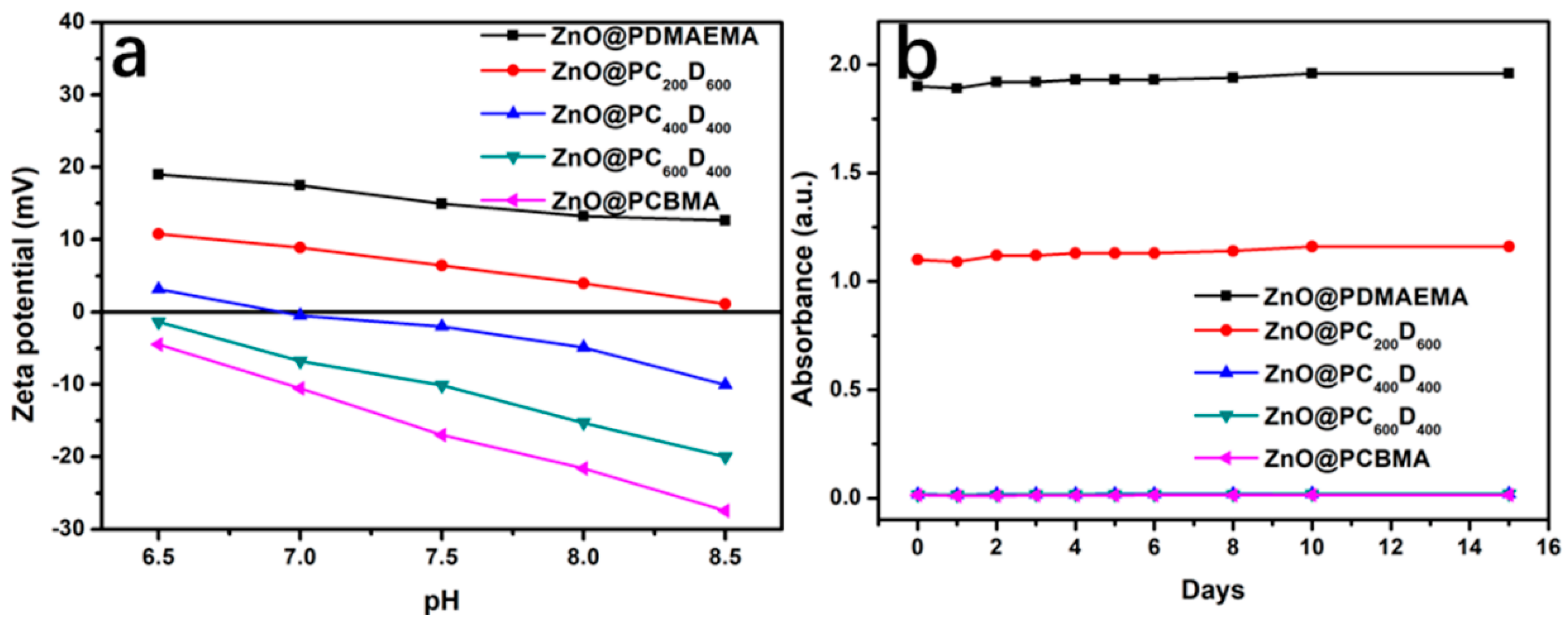
2.4. Stability Tests of the ZnO-Based NDDSs
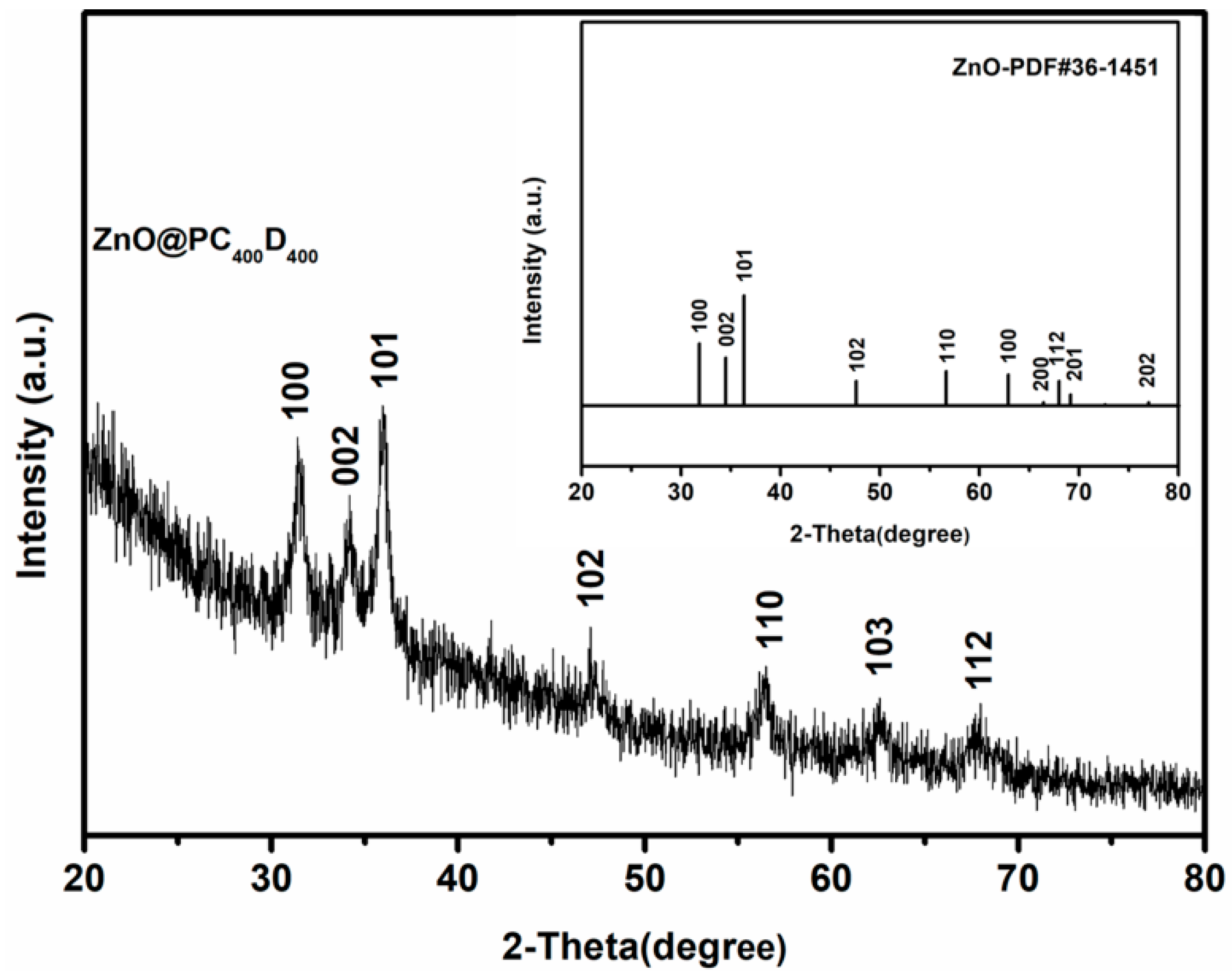
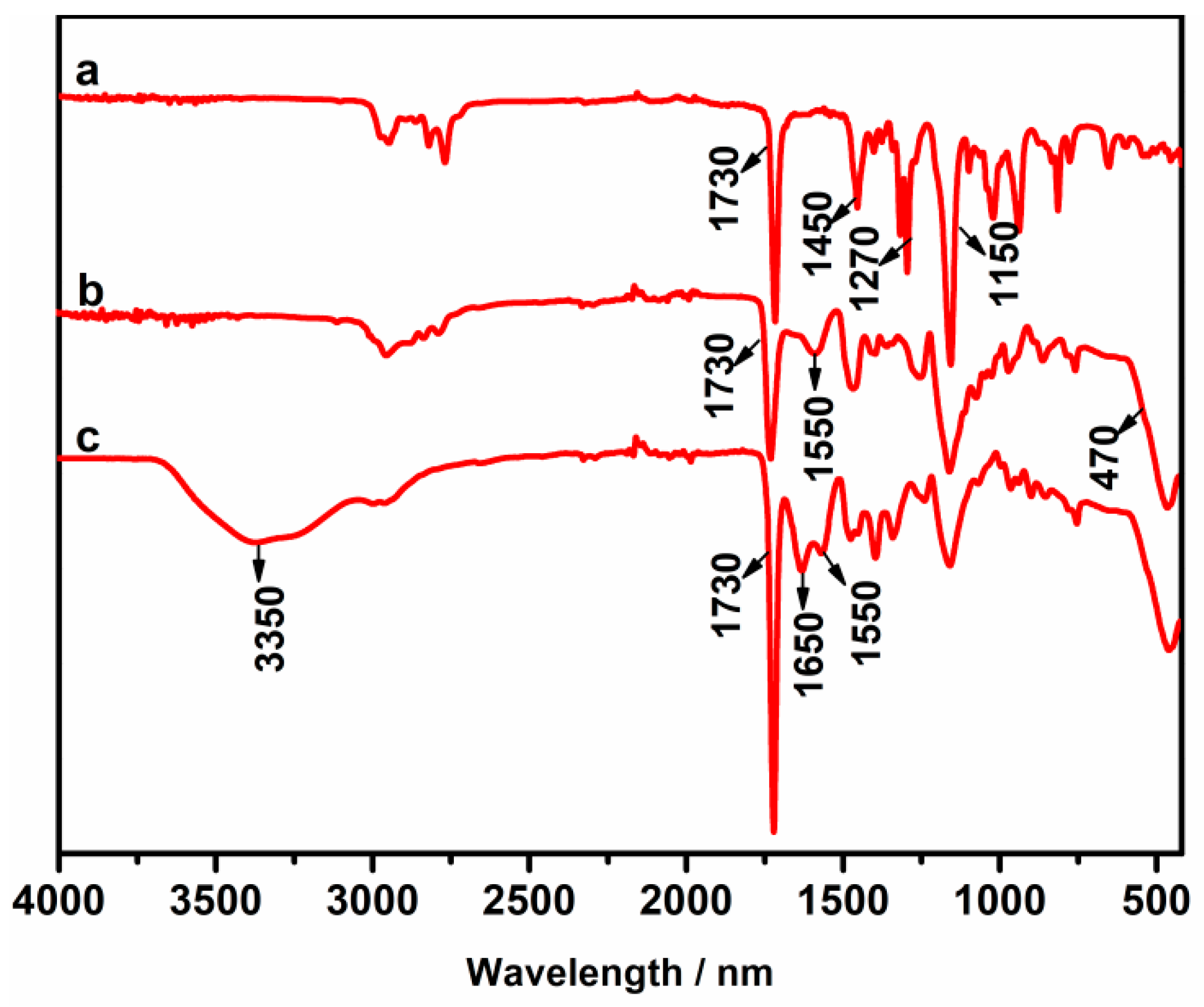
2.5. Characterization
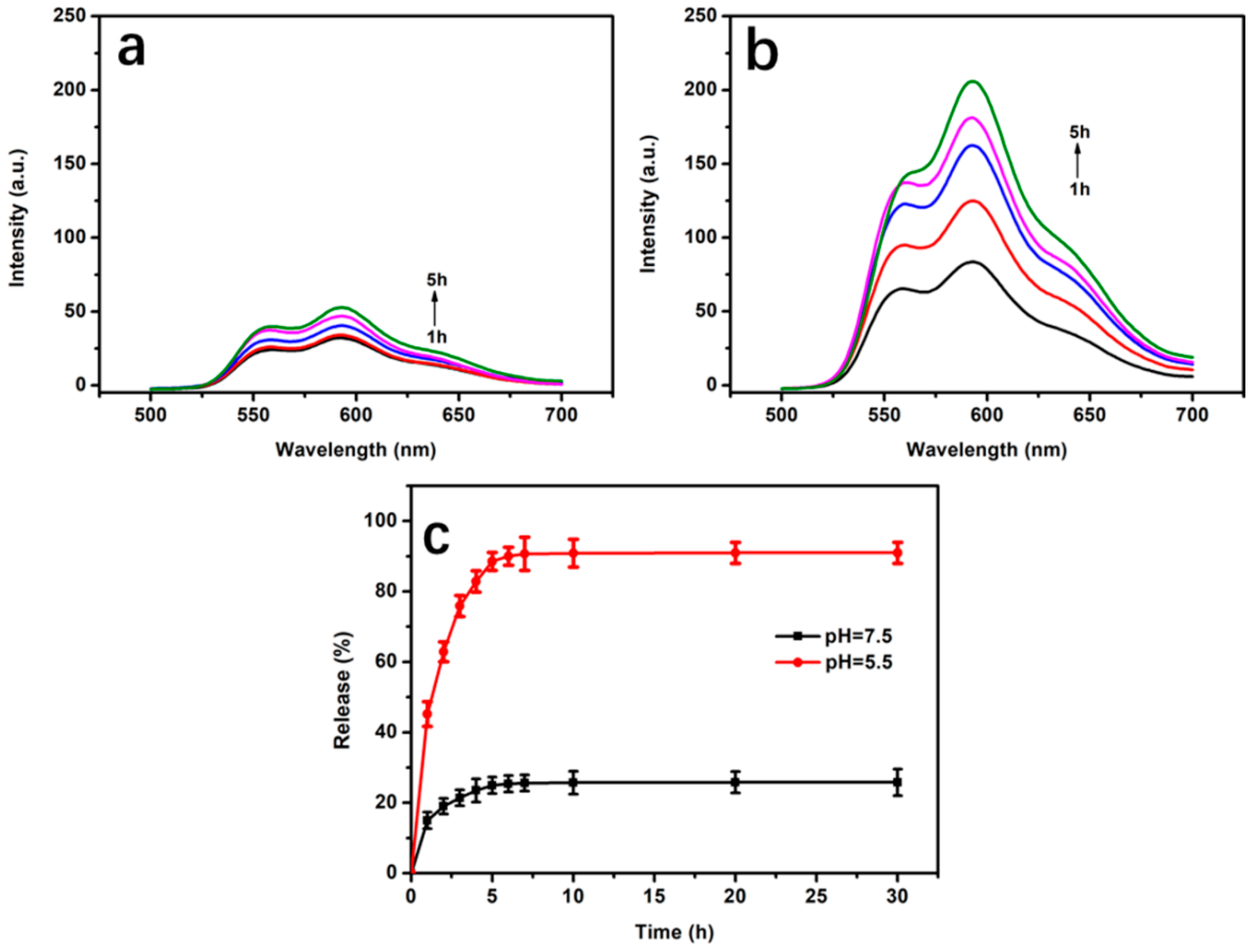
2.6. Drug Loading and Release
2.7. Confocal Microscopy
2.8. Cytotoxicity Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, R.; Tsai, W.B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers 2018, 10, 418. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidi, K.; Neubauer, H.A.; Moriggl, R.; Jahanban-Esfahlan, R.; Javaheri, T. Tumor target amplification: implications for nano drug delivery systems. J. Control. Release 2018, 275, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nano-Graphene Oxide for Delivery of Water Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Del, P.P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; Jm, D.L.F.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, Z.; Zhang, L.; Zhao, J.; Zhang, Q.; Peng, L.; Zhang, W.; Ye, M.; Zou, H. Facile synthesis of zwitterionic polymer-coated core-shell magnetic nanoparticles for highly specific capture of N-linked glycopeptides. Nanoscale 2015, 7, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Cheng, T.; Zhang, Y.; Liu, J.; Ding, Y.; Zhen, J.; Shen, W.; Xu, Y.; Yang, W.; Niu, P. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2017, 65, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shao, J.; Jin, Q.; Wei, Q.; Tang, J.; Ji, J. Zwitterionic phosphorylcholine as a better ligand for gold nanorods cell uptake and selective photothermal ablation of cancer cells. Chem. Commun. 2010, 46, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2011, 42, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Li, B.; Chen, D.; Dong, X.; Wang, Y.; Gu, Y. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials 2015, 53, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lu, M.; Rutkowski, B.; Dai, X.Y.; Yang, Y.; Taccardi, N.; Stachewicz, U.; Czyrska-Filemonowicz, A.; Hüser, N.; Boccaccini, A.R. ZnO quantum dots modified bioactive glass nanoparticles with pH-sensitive release of Zn ions, fluorescence, antibacterial and osteogenic properties. J. Mater. Chem. B 2016, 4, 7936–7949. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Huang, X.; Du, X. pH- and Redox-Triggered Synergistic Controlled Release of ZnO-Gated Hollow Mesoporous Silica Drug Delivery System. J. Mater. Chem. B 2015, 3, 1426–1432. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.F.; Feng, J. Multifunctional Mesoporous Silica Nanoparticles Based on Charge-Reversal Plug-Gate Nanovalves and Acid-Decomposable ZnO Quantum Dots for Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 26666–26673. [Google Scholar] [CrossRef] [PubMed]
- Othman, B.A.; Greenwood, C.; Abuelela, A.F.; Bharath, A.A.; Chen, S.; Theodorou, I.; Douglas, T.; Uchida, M.; Ryan, M.; Merzaban, J.S. Correlative Light—Electron Microscopy Shows RGD—Targeted ZnO Nanoparticles Dissolve in the Intracellular Environment of Triple Negative Breast Cancer Cells and Cause Apoptosis with Intratumor Heterogeneity. Adv. Healthc. Mater. 2016, 5, 1310–1325. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Misra, R.D. Structural and physicochemical aspects of silica encapsulated ZnO quantum dots with high quantum yield and their natural uptake in HeLa cells. J. Biomed. Mater. Res. A 2014, 102, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, A.; Kompch, A.; Mackert, V.; Liebscher, C.H.; Winterer, M. Interaction of L-cysteine with ZnO: Structure, surface chemistry and optical properties. Langmuir 2015, 31, 5701–5711. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Matsuyama, K.; Maeda, Y.K.; Matsuda, T.; Okuyama, T.; Muto, H. Formation of poly(methyl methacrylate)-ZnO nanoparticle quantum dot composites by dispersion polymerization in supercritical CO 2. J. Supercrit. Fluids 2015, 103, 83–89. [Google Scholar] [CrossRef]
- Han, X.; Huang, S.; Wang, Y.; Shi, D. Design and development of anisotropic inorganic/polystyrene nanocomposites by surface modification of zinc oxide nanoparticles. Mater. Sci. Eng. C 2016, 64, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Lu, X.; Zhang, Q. Enhancing luminescence of ZnO quantum dots by PEG and oleic acid via a sol–gel method. J. Mater. Sci. Mater. Electron. 2015, 26, 1113–1118. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Guo, Y.; Qi, W.; Qu, F.; Sun, F.; Zhao, H.; Zhu, G. Acid degradable ZnO quantum dots as a platform for targeted delivery of an anticancer drug. J. Mater. Chem. 2011, 21, 13406–13412. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots-Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W. ZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials 2010, 31, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Shouju, W.; Zhaogang, T.; Peng, H.; Dingbin, L.; Ying, L.; Ying, T.; Jing, S.; Yanjun, L.; Huangxian, J.; Xiaoyuan, C. Photothermal Therapy: Reversibly Extracellular pH Controlled Cellular Uptake and Photothermal Therapy by PEGylated Mixed-Charge Gold Nanostars. Small 2015, 11, 1801–1810. [Google Scholar]
- Li, H.J.; Du, J.Z.; Liu, J.; Du, X.J.; Shen, S.; Zhu, Y.H.; Wang, X.; Ye, X.; Nie, S.; Wang, J. Smart Superstructures with Ultrahigh pH-Sensitivity for Targeting Acidic Tumor Microenvironment: Instantaneous Size Switching and Improved Tumor Penetration. ACS Nano 2016, 10, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; Mcsheehy, P.M.J.; Griffiths, J.R.; Bashford, C.L. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef]
- Onyango, J.O.; Chung, M.S.; Eng, C.H.; Klees, L.M.; Langenbacher, R.; Yao, L.; An, M. Noncanonical Amino Acids to Improve the pH Response of pHLIP Insertion at Tumor Acidity. Angew. Chem. Int. Ed. 2015, 54, 3658–3663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, P.; Bernards, M.T.; Deng, B. Modification of polysulfone (PSF) hollow fiber membrane (HFM) with zwitterionic or charged polymers. Ind. Eng. Chem. Res. 2017, 56, 7576–7584. [Google Scholar] [CrossRef]
- Li, B.; Yuan, Z.; Peng, Z.; Sinclair, A.; Jain, P.; Kan, W.; Tsao, C.; Xie, J.; Hung, H.C.; Lin, X. Zwitterionic Nanocages Overcome the Efficacy Loss of Biologic Drugs. Adv. Mater. 2018, 30, 1705728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Tsao, C.; Liu, S.; Jain, P.; Sinclair, A.; Hung, H.C.; Bai, T.; Wu, K.; Jiang, S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. USA 2015, 112, 12046–12051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Xu, G.; Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.T. pH-Sensitive Zwitterionic Polymer as an Antimicrobial Agent with Effective Bacterial Targeting. ACS Biomater. Sci. Eng. 2017, 4, 40–46. [Google Scholar] [CrossRef]
- Chen, K.; Hu, F.; Gu, H.; Xu, H. Tuning of surface protein adsorption by spherical mixed charged silica brushes (MCB) with zwitterionic carboxybetaine component. J. Mater. Chem. B 2017, 5, 435–443. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Ševců, V.; Adam, P.; Špačková, B.; Hegnerová, K.; de los Santos Pereira, A.; Rodriguez-Emmenegger, C.; Riedel, T.; Houska, M.; Brynda, E.; et al. Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms: Functionalization capacity, biorecognition capability and resistance to fouling from undiluted biological media. Biosens. Bioelectron. 2014, 51, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wan, J.; Sun, L.; Li, Y.; Guo, J.; Wang, C. Zinc finger-inspired nanohydrogels with glutathione/pH triggered degradation based on coordination substitution for highly efficient delivery of anti-cancer drugs. J. Control. Release 2016, 225, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.X.; Ma, Y.Y.; Zhao, W.; Cao, H.M.; Kong, J.L.; Xiong, H.M.; Möhwald, H. ZnO-Based Nanoplatforms for Labeling and Treatment of Mouse Tumors without Detectable Toxic Side Effects. ACS Nano 2016, 10, 4294–4300. [Google Scholar] [CrossRef] [PubMed]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Xiong, H.M.; Zhao, X.; Chen, J.S. Inorganic Nanocomposites PEO−ZnO and PEO−ZnO−LiClO 4 Films. J. Phy. Chem. B 2001, 105, 10169–10174. [Google Scholar] [CrossRef]




| Sample | CBMA/DMAEMA (mg/mg) | Dh1 (nm) | PDI 1 |
|---|---|---|---|
| ZnO@PDMAEMA | 0/800 | 10.1 | 0.272 |
| ZnO@PC200D600 | 200/600 | 10.6 | 0.318 |
| ZnO@PC400D400 | 400/400 | 11.7 | 0.386 |
| ZnO@PC600D200 | 600/200 | 11.6 | 0.324 |
| ZnO@PCBMA | 800/0 | 11.9 | 0.354 |
| Sample | Zeta-Potential (mV) (pH = 7.5) | Zeta-Potential (mV) (pH = 6.5) |
|---|---|---|
| ZnO@PDMAEMA | 14.94 | 18.99 |
| ZnO@PC200D600 | 6.42 | 10.78 |
| ZnO@PC400D400 | −2.01 | 3.14 |
| ZnO@PC600D200 | −10.12 | −1.38 |
| ZnO@PCBMA | −16.98 | −4.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; He, L.; Yu, B.; Chen, Y.; Shen, Y.; Cong, H. ZnO Quantum Dots Modified by pH-Activated Charge-Reversal Polymer for Tumor Targeted Drug Delivery. Polymers 2018, 10, 1272. https://doi.org/10.3390/polym10111272
Wang Y, He L, Yu B, Chen Y, Shen Y, Cong H. ZnO Quantum Dots Modified by pH-Activated Charge-Reversal Polymer for Tumor Targeted Drug Delivery. Polymers. 2018; 10(11):1272. https://doi.org/10.3390/polym10111272
Chicago/Turabian StyleWang, Yifan, Liang He, Bing Yu, Yang Chen, Youqing Shen, and Hailin Cong. 2018. "ZnO Quantum Dots Modified by pH-Activated Charge-Reversal Polymer for Tumor Targeted Drug Delivery" Polymers 10, no. 11: 1272. https://doi.org/10.3390/polym10111272