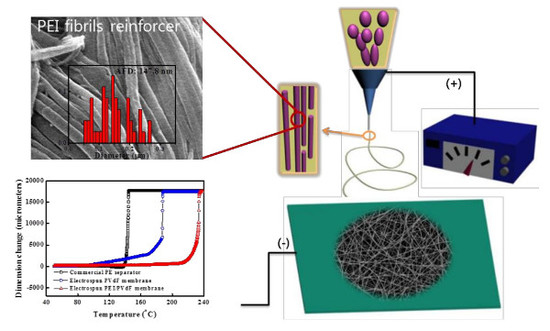
Reinforced PEI/PVdF Multicore-Shell Structure Composite Membranes by Phase Prediction on a Ternary Solution
Abstract
:1. Introduction
2. Theory and Computation
3. Experimental Details
3.1. Spinning Solution
3.2. Membrane Preparation
3.3. Characterization
4. Results and Discussion
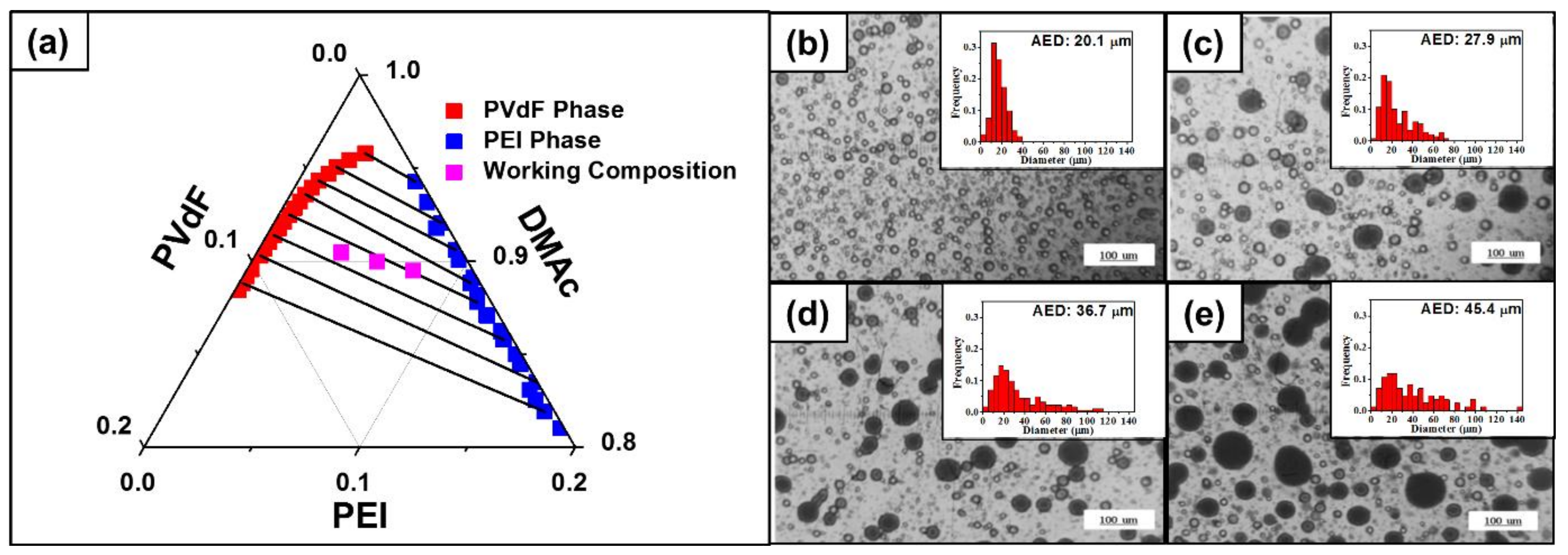
4.1. Phase Separation in Ternary Solution
4.2. Diameter of Electrospun Fiber
4.3. Morphology of the Fiber
4.4. PEI as a Multiple-Core Fibril
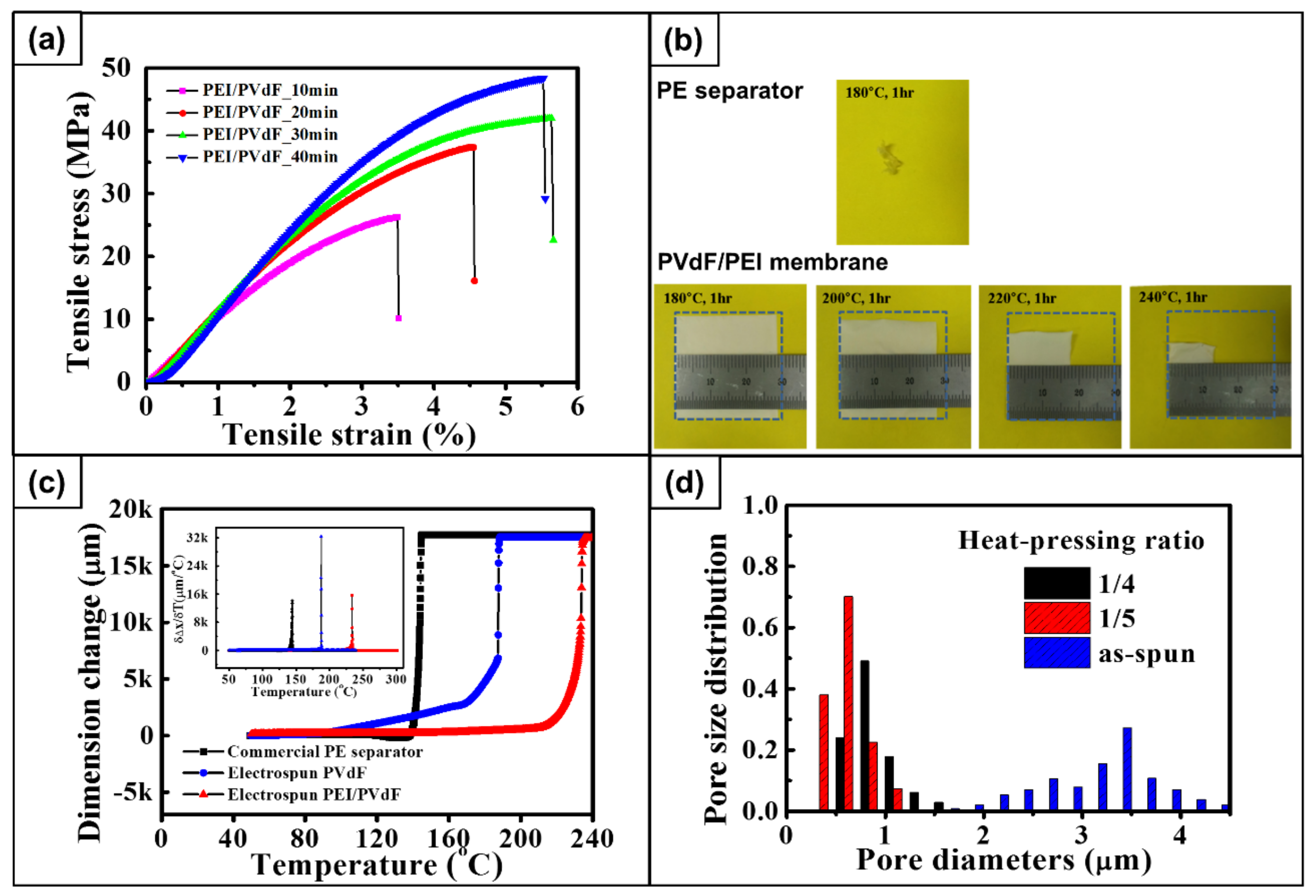
4.5. Mechanical Properties
4.6. Thermal Stability
4.7. Textural Properties
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ziabicki, A. Fundamentals of Fibre Formation: The Science of Fibre Spinning and Drawing; Wiley: London, UK, 1976. [Google Scholar]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Hunley, M.T.; Long, T.E. Electrospinning functional nanoscale fibers: A perspective for the future. Polym. Int. 2008, 57, 385–389. [Google Scholar] [CrossRef]
- Bazilevsky, A.V.; Yarin, A.L.; Megaridis, C.M. Co-electrospinning of Core−Shell Fibers Using a Single-Nozzle Technique. Langmuir 2007, 23, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanathan, P.; Prabu, A.A.; Kim, K.J. Electrospun polyvinylidene fluoride-polyoctafluoropentyl acrylate blend based piezocapacitive pressure sensors. Macromol. Res. 2016, 24, 670–674. [Google Scholar] [CrossRef]
- Lee, B.; Shafiq, M.; Jung, Y.; Park, J.-C.; Kim, S.H. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a co-culture system. Macromol. Res. 2016, 24, 131–142. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.M.; Ma, X.T.; Shi, J.L.; Zhu, B.K.; Zhu, L.P. Gel polymer electrolytes based on active PVDF separator for lithium ion battery. I: Preparation and property of PVDF/poly(dimethylsiloxane) blending membrane. J. Membr. Sci. 2011, 379, 397–402. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J.R.; Ahn, Y.R.; Jo, S.M.; Cairns, E.J. Characterization of Electrospun PVdF Fiber-Based Polymer Electrolytes. Chem. Mater. 2006, 19, 104–115. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim. Acta 2004, 50, 69–75. [Google Scholar] [CrossRef]
- Lee, S.W.; Choi, S.W.; Jo, S.M.; Chin, B.D.; Kim, D.Y.; Lee, K.Y. Electrochemical properties and cycle performance of electrospun poly(vinylidene fluoride)-based fibrous membrane electrolytes for Li-ion polymer battery. J. Power Sources 2006, 163, 41–46. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J.R.; Jo, S.M.; Lee, W.S.; Kim, Y.R. Electrochemical and Spectroscopic Properties of Electrospun PAN-Based Fibrous Polymer Electrolytes. J. Electrochem. Soc. 2005, 152, A989–A995. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Characterization and Properties of P(VdF-HFP)-Based Fibrous Polymer Electrolyte Membrane Prepared by Electrospinning. J. Electrochem. Soc. 2005, 152, A295–A300. [Google Scholar] [CrossRef]
- Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, Y.R. An Electrospun Poly(vinylidene fluoride) Nanofibrous Membrane and Its Battery Applications. Adv. Mater. 2003, 15, 2027–2032. [Google Scholar] [CrossRef]
- Bijwe, J.; Rattan, R. Carbon fabric reinforced polyetherimide composites: Optimization of fabric content for best combination of strength and adhesive wear performance. Wear 2007, 262, 749–758. [Google Scholar] [CrossRef]
- Bastida, S.; Eguiazábal, J.I.; Nazábal, J. Structure and physical properties of reprocessed poly(ether imide). J. Appl. Polym. Sci. 1997, 63, 1601–1607. [Google Scholar] [CrossRef]
- Mark, H.F.; Bikales, N.M.; Overberger, C.G.; Nenges, G.; Kroschwitz, J.I. Encyclopedia of Polymer Science and Engineering; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Hou, M.; Ye, L.; Lee, H.J.; Mai, Y.W. Manufacture of a carbon-fabric-reinforced polyetherimide (CF/PEI) composite material. Compos. Sci. Technol. 1998, 58, 181–190. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Liu, S.W.; Chi, Z.G.; Peng, X.F.; Lin, Y.Q.; Xu, J.R. Reinforcing and toughening on poly(ether imide) by a novel thermo tropic liquid crystalline poly(ester-imide-ketone) with low content. Polym. Eng. Sci. 2009, 49, 2046–2053. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X. Influence of addition of silica particles on reaction-induced phase separation and properties of epoxy/PEI blends. Compos. Part B Eng. 2011, 42, 2163–2169. [Google Scholar] [CrossRef]
- Chen, W.M.; Tao, Z.Q.; Fan, L.; Yang, S.Y.; Jiang, W.G.; Wang, J.F.; Xiong, Y.L. Effect of poly(etherimide) chemical structures on the properties of epoxy/poly(etherimide) blends and their carbon fiber-reinforced composites. J. Appl. Polym. Sci. 2011, 119, 3162–3169. [Google Scholar] [CrossRef]
- Boom, R.M.; Vandenboomgaard, T.; Smolders, C.A. Equilibrium Thermodynamics of a Quaternary Membrane-Forming System with Two Polymers. 1. Calculations. Macromolecules 1994, 27, 2034–2040. [Google Scholar] [CrossRef]
- Baik, K.J.; Kim, J.Y.; Lee, H.K.; Kim, S.C. Liquid–liquid phase separation in polysulfone/polyethersulfone/N-methyl-2-pyrrolidone/water quaternary system. J. Appl. Polym. Sci. 1999, 74, 2113–2123. [Google Scholar] [CrossRef]
- Yilmaz, L.; Mchugh, A.J. Analysis of nonsolvent–solvent–polymer phase diagrams and their relevance to membrane formation modeling. J. Appl. Polym. Sci. 1986, 31, 997–1018. [Google Scholar] [CrossRef]
- Kumar, R.; Sumpter, B.G.; Muthukumar, M. Enhanced Phase Segregation Induced by Dipolar Interactions in Polymer Blends. Macromolecules 2014, 47, 6491–6502. [Google Scholar] [CrossRef]
- Hsu, C.C.; Prausnit, J.M. Thermodynamics of Polymer Compatibility in Ternary Systems. Macromolecules 1974, 7, 320–324. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Penning, J.P.; Puyenbroek, R.; Willems, G.J. High Performance Thermoplastic Compositions with Improved Melt Flow Behavior. U.S. Patent 6,417,255, 2002. [Google Scholar]
- Chae, D.W.; Kim, M.H.; Kim, B.C. Temperature dependence of the rheological properties of poly(vinylidene fluoride)/dimethyl acetamide solutions and their electrospinning. Korea-Aust. Rheol. J. 2010, 22, 229–234. [Google Scholar]
- Cai, T.; Wang, R.; Yang, W.J.; Lu, S.J.; Neoh, K.G.; Kang, E.T. Multi-functionalization of poly(vinylidene fluoride) membranes via combined “grafting from” and “grafting to” approaches. Soft Matter 2011, 7, 11133–11143. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]


| Component | Dispersion (MPa1/2) | Polar (MPa1/2) | Hydrogen Bonding (MPa1/2) |
|---|---|---|---|
| DMAc | 16.8 | 11.5 | 10.2 |
| PVdF | 17.0 | 12.1 | 10.2 |
| PEI | 19.6 | 7.6 | 9.0 |
| Samples | Tensile Strength (MPa) | Tensile Modulus (MPa) | Strain-to-Failure (%) |
|---|---|---|---|
| PEI/PVdF_10min | 26.3 | 1039 | 3.5 |
| PEI/PVdF_20min | 37.4 | 1261 | 4.5 |
| PEI/PVdF_30min | 42.0 | 1328 | 5.7 |
| PEI/PVdF_40min | 48.4 | 1475 | 5.5 |
| Samples | App. Porosity (%) | Mean Flow Pore Size (μm) | Average Gurley Number (s/100 cc) | Uptake with Electrolyte (%) |
|---|---|---|---|---|
| As-electrospun | 89.1 | 3.26 | 0.79 | 475 |
| Post-treated | 58.3 | 0.81 | 6.29 | 365 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, J.; Park, S.; Kim, D.Y.; Joh, H.-I.; Kim, J.M.; Lee, S.; Jo, S.M. Reinforced PEI/PVdF Multicore-Shell Structure Composite Membranes by Phase Prediction on a Ternary Solution. Polymers 2018, 10, 436. https://doi.org/10.3390/polym10040436
Chae J, Park S, Kim DY, Joh H-I, Kim JM, Lee S, Jo SM. Reinforced PEI/PVdF Multicore-Shell Structure Composite Membranes by Phase Prediction on a Ternary Solution. Polymers. 2018; 10(4):436. https://doi.org/10.3390/polym10040436
Chicago/Turabian StyleChae, Jihye, Sejoon Park, Dong Young Kim, Han-Ik Joh, Jong Man Kim, Sungho Lee, and Seong Mu Jo. 2018. "Reinforced PEI/PVdF Multicore-Shell Structure Composite Membranes by Phase Prediction on a Ternary Solution" Polymers 10, no. 4: 436. https://doi.org/10.3390/polym10040436