Double-Dosing and Other Dangers with Non-Prescription Medicines: Pharmacists’ Views and Experiences
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Views on the Reclassification of the Shingles Vaccine and Sildenafil
3.2. Views on the Reclassification of Paracetamol and Ibuprofen Liquids to General Sales
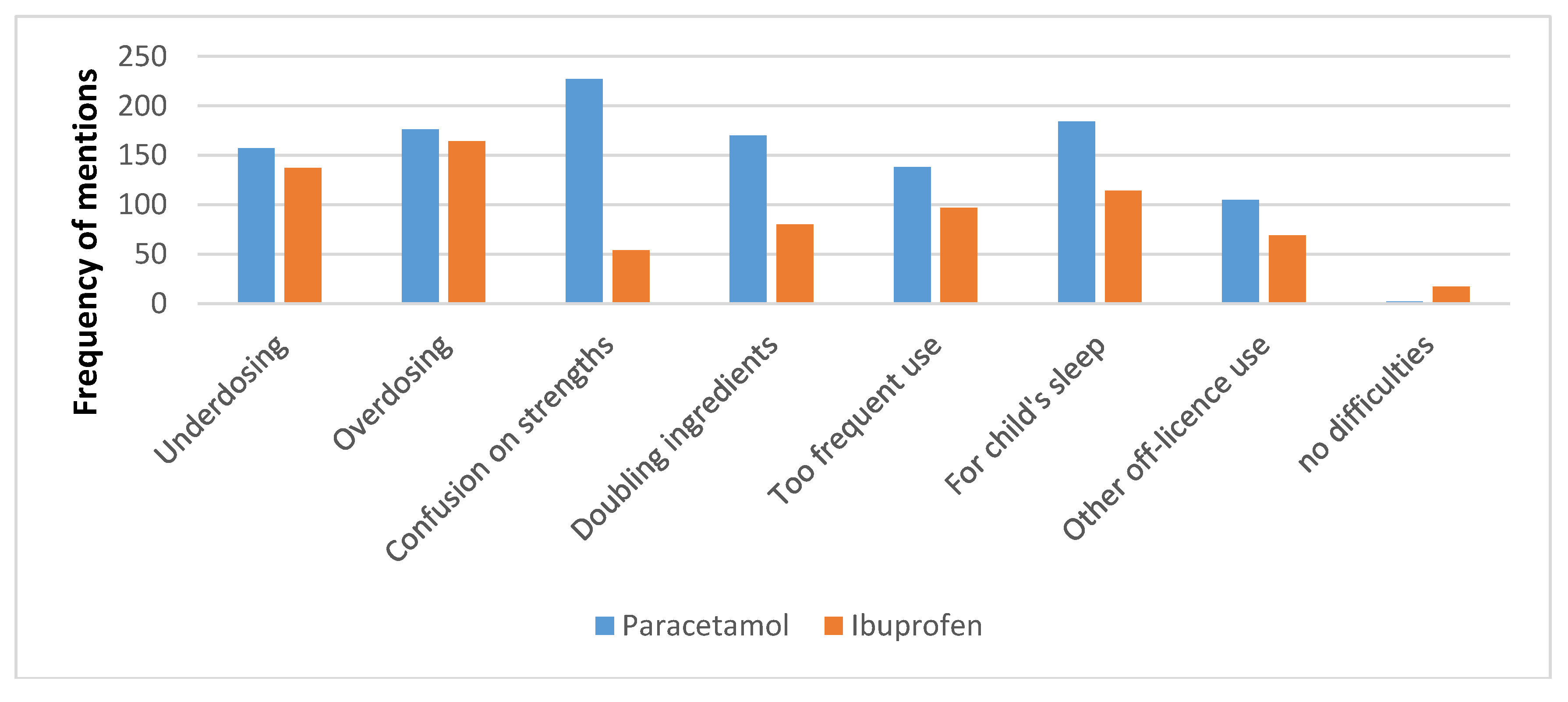
3.3. Experiences and Advice with Paracetamol and Ibuprofen Liquids
“Parents don’t often know the correct amount of paracetamol to give to their kids and different strengths of paracetamol make it even more confusing.”“People not realising different brands [are] same thing (Panadol, Pamol), people not realising how dangerous paracetamol is in overdose, considered “safe” cos it’s common.”“Some parents dose paracetamol around the clock e.g., q4h = six doses in 24 h. Many parents think orange flavour is stronger, strawberry is weaker, but depends on brand!”“Double-dosing for more pain relief, dosing more frequently even with well-educated patients.”
“Using as first line pain relief for very young babies—Nurofen has been aggressively marketed to parents of young babies.”
“Very common for parents to not update dose according to weight. We try to advise [at] every visit/purchase.”“Giving information on correct dose and frequency. Pointing out that it will only help child to sleep if they have pain or fever relieved by the paracetamol.”“We remind people … not to double-up. Have had incidences of wrong doses, doubling-up, confusion over use, strength. We ask for children’s weight to check paracetamol and ibuprofen doses.”
“People always want to use it [paracetamol] for sleep.”
“Customers think that paracetamol is a cure for lots of children’s medical problems.”“Use for cough or sleep aid. If child is grumpy may use paracetamol. When prescription given out or sold OTC, we always counsel.”“Purchasing for vomiting + diarrhoea [ibuprofen].”
3.4. Views on the Reclassification of Omeprazole, Naproxen, and Nasal Oxymetazoline to General Sales
3.5. Experiences with Omeprazole
“Patients have problems differentiating between angina and heartburn.”“When patient thinks it’s heartburn but actually was angina symptoms. Told to get to doctor, Nitrolingual [glyceryl trinitrate] prescribed.”“… patient was having heart attack, treating with antacids and then came in for omeprazole but had pain in shoulders, jaw, sent to [doctor], ended up in ambulance with heart attack, came back and thanked me.”“A customer wanted some but on discussion of symptoms sent to GP as symptoms suggestive of MI. Correct, had triple bypass surgery in subsequent month.”“Patients presenting with cardiac symptoms but thinking it’s indigestion; patients over 50 [years of age] with indigestion not going to [doctor] and buying antacids; a couple of instances where we have had to call ambulance [for possible] heart attack.”
“When questioning patient, found out had serious problem, [doctor] in UK had prescribed omeprazole but I sent him to a local doctor—turned out he had throat cancer, now on chemotherapy and had throat surgery. This would not have been picked up by selling product in supermarket.”“Overuse—had a customer that we referred and turned out to be stomach cancer. Life saved!”“Stopped young male patient self-medicating and had gastroscopy- gastric cancer discovered and treated.”“Blood disorders and two cases of identifying stomach cancers.”“Long-term epigastric pain ignored and/or self-treated; patient had cancer and died.”
“Regular patient who comes in regularly for omeprazole, we advised them to visit doctor as he was increasing dose of OTC omeprazole—eventually found out he had an H. pylori infection.”“… increased use and when advised to go to the doctor, had a perforation.”“Regular use without diagnosis from [doctor]—bleeding stomach ulcer resulting in hospital admin.”“Had a few patients that needed to be referred to [doctor] with peptic ulcer concern.”
“Advertised on TV and misinterpretation of ad symptoms frequent—often [we] check history and find [that the patient is] already on an H2 antagonist or PPI.”“People taking NSAIDS (four or more [patients]) and not consulting doctor.”
“Customers think it is appropriate to take this OTC pretty regularly. Also had a case of a lady buying it because the doctor wouldn’t prescribe it as the doctor wanted her to have a gastroscopy.”
“… had a customer try to buy it for 12-week-old twin babies then changed her story so that she could just purchase it, still with the intention of being able to give to her friend’s babies who were having difficulties. Sale refused on all grounds. Explained dangers and also highlighted to other local pharmacies just in case she still attempted to get [it].”
3.6. Experiences with Naproxen and Other NSAIDs
“People not realising already taking another NSAID e.g, Nurofen [non-prescription ibuprofen brand] or Voltaren [diclofenac] + Nurofen. [This] happens quite frequently.”“Misunderstanding of clients of the difference and similarities of anti-inflammatories. It is not unusual to find patients taking [two or more].”
“Patients taking diclofenac wanting to buy naproxen for period pain, Nurofen cold and flu [ibuprofen with a decongestant], with Nuromol [paracetamol and ibuprofen].”
“Pregnant lady taking NSAIDs for months that she had bought from a supermarket.”“Several times a year find people with stomach ulcers want to buy NSAIDs.”“Patients allergic to aspirin didn’t realise could be allergic to other NSAIDs. Patients with stomach ulcers wanting to buy, not realising risks.”“Older diabetic patients trying to buy multiple boxes, same with hypertensive patients.”“Only yesterday patient with cardiac surgery history wanted naproxen because recommended as safe by a nursing friend.”
“On questioning patient is on blood thinning meds, had previous or currently has stomach issues (ulcers).”“Elderly customers asking for NSAIDs when they have [gastro-intestinal] problems or cardiovascular disease or both.”“Often this type of medication is requested by patients on many meds and [those who] have chronic pain issues. Often on PPIs.”
“Medicine interactions is the most common issue—many people think that NSAIDs are ok with all meds especially because they can buy Nurofen [ibuprofen] from the supermarket!”“Triple whammy a number of times.”
“Sports people using it before sports—potential for kidney damage—refer to Panadol [paracetamol], using anti-inflammatories before 48 h after injury.”
“Elderly patient asking for NSAIDs (diclofenac), the doctor had previously stopped it 2 years ago due to side effects, but patient is unable to see why we refused sale when she ‘really needs it’. Patient taking Naproxen and wanting diclofenac. Patient wanting NSAIDs at first injury (i.e., within 48 h). Patient wanting NSAIDs and hasn’t trialed any other type of pain relief. Asthmatic patient wanting NSAIDs.”
“Blasé attitude about this often and how much to give. People believe it is very safe to use, anywhere, anytime.”“Continually using NSAIDs causing stomach bleeds, advised alternative pain relief.”
“Being used to treat gout but patient not on preventive medication—sent to [doctor], explained need to lower uric acid levels.”
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lalonde, L.; Tsuyuki, R.T.; Landry, E.; Taylor, J. Results of a national survey on otc medicines, part 2: Do pharmacists support switching prescription agents to over-the-counter status? Can. Pharm. J. 2012, 145, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Erwin, J.O.; Britten, N.; Jones, R. Pharmacists and deregulation: The case of h2-antagonists. J. Soc. Adm. Pharm. 1996, 13, 150–158. [Google Scholar]
- Madhavan, S.; Schondelmeyer, S.W. Attitudes of pharmacists toward rx-to-otc switches and their effect on pharmacists’ overall judgment of switch appropriateness. J. Pharm. Mark. Manag. 1990, 4, 3–25. [Google Scholar] [CrossRef]
- Paudyal, V.; Hansford, D.; Cunningham, S.; Stewart, D. Pharmacists’ adoption into practice of newly reclassified medicines from diverse therapeutic areas in scotland: A quantitative study of factors associated with decision-making. Res. Soc. Adm. Pharm. 2014, 10, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Guignard, A.P.; Couray-Targe, S.; Colin, C.; Chamba, G. Economic impact of pharmacists’ interventions with nonsteroidal antiinflammatory drugs. Ann. Pharmacother. 2003, 41, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, C.; Hämmerlein, A.; Griese, N.; Schulz, M. Nature and frequency of drug-related problems in self-medication (over-the-counter drugs) in daily community pharmacy practice in germany. Pharmacoepidemiol. Drug Saf. 2012, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Emmerton, L.M.; Taylor, R.; Werner, J.; Benrimoj, S.I. Non-prescription medicines and australian community pharmacy interventions: Rates and clinical significance. Int. J. Pharm. Pract. 2011, 19, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Emmerton, L.; Chaw, X.Y.; Kelly, F.; Kairuz, T.; Marriott, J.; Wheeler, A. Management of children’s fever by parents and caregivers: Practical measurement of functional health literacy. J. Child Health Care 2013, 18, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Catlin, J.R.; Pechmann, C.; Brass, E.P. Dangerous double dosing: How naive beliefs can contribute to unintentional overdose with over-the-counter drugs. J. Public Policy Mark. 2015, 34, 194–209. [Google Scholar] [CrossRef]
- Gauld, N.J.; Shaw, J.P.; Emmerton, L.M.; Pethica, B.D. Surveillance of a recently switched non-prescription medicine (diclofenac) using a pharmacy-based approach. Pharmacoepidemiol. Drug Saf. 2000, 9, 207–214. [Google Scholar] [CrossRef]
- Rogers, P.J.; Wood, S.M.; Garrett, E.L.; Krykant, S.P.; Haddington, N.J.; Hayhurst, J.; Player, G.R.H. Use of nonprescription topical steroids: Patients’ experiences. Br. J. Dermatol. 2005, 152, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Gauld, N.J.; Kelly, F.S.; Emmerton, L.M.; Buetow, S.A. Widening consumer access to medicines: A comparison of prescription to non-prescription medicine switch in Australia and New Zealand. PLoS ONE 2015, e0119011. [Google Scholar] [CrossRef] [PubMed]
- Gauld, N.J.; Kelly, F.S.; Kurosawa, N.; Bryant, L.J.M.; Emmerton, L.M.; Buetow, S.A. Widening consumer access to medicines through switching medicines to non-prescription: A six country comparison. PLoS ONE 2014, 9, e107726. [Google Scholar] [CrossRef] [PubMed]
- Hansford, D.; Cunningham, S.; John, D.; McCaig, D.; Stewart, D. Community pharmacists’ views, attitudes and early experiences of over-the-counter simvastatin. Pharm. World Sci. 2007, 29, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.; Collier, J.; Birchley, N.; Neil, K.; Rodgers, S.; McIntyre, J.; Choonara, I.; Avery, A. An examination of the risk management issues in the handling at home of over-the-counter medicines purchased for children. Pharm. J. 2003, 271, 209–213. [Google Scholar]
- Bilenko, N.; Tessler, H.; Okbe, R.; Press, J.; Gorodischer, R. Determinants of antipyretic misuse in children up to 5 years of age: A cross-sectional study. Clin. Ther. 2006, 28, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, V.; Hansford, D.; Cunningham, S.; Stewart, D. Pharmacists’ Perceived integration into practice of over-the-counter simvastatin five years post reclassification. Int. J. Clin Pharm. 2012, 34, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Gauld, N.; Kelly, F.; Shaw, J. Is non-prescription oseltamivir availability under strict criteria workable? A qualitative study in New Zealand. J. Antimicrob. Chemother. 2011, 66, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Braund, R.; Henderson, E.; McNab, E.; Sarten, R.; Wallace, E.; Gauld, N. Pharmacist-only trimethoprim: Pharmacist satisfaction on their training and the impact on their practice. Int. J. Clin. Pharm. 2016, 38, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Paracetamol Fact Sheet. Available online: http://www.poisons.co.nz/factp.php?f=33 (accessed on 29 April 2018).
- Bardage, C.; Westerlund, T.; Barzi, S.; Bernsten, C. Non-prescription medicines for pain and fever-a comparison of recommendations and counseling from staff in pharmacy and general sales stores. Health Policy 2013, 110, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cham, E.; Hall, L.; Ernst, A.A.; Weiss, S.J. Awareness and use of over-the-counter pain medications: A survey of emergency department patients. South Med. J. 2002, 95, 529–535. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauld, N.; Sullivan, T. Double-Dosing and Other Dangers with Non-Prescription Medicines: Pharmacists’ Views and Experiences. Pharmacy 2018, 6, 59. https://doi.org/10.3390/pharmacy6030059
Gauld N, Sullivan T. Double-Dosing and Other Dangers with Non-Prescription Medicines: Pharmacists’ Views and Experiences. Pharmacy. 2018; 6(3):59. https://doi.org/10.3390/pharmacy6030059
Chicago/Turabian StyleGauld, Natalie, and Tracey Sullivan. 2018. "Double-Dosing and Other Dangers with Non-Prescription Medicines: Pharmacists’ Views and Experiences" Pharmacy 6, no. 3: 59. https://doi.org/10.3390/pharmacy6030059



