Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs—Design, Fabrication, and Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
2.2. Cream Formulation
2.3. Franz Diffusion Cell Study
2.3.1. Skin Preparation
2.3.2. Sample Collection
2.4. Transdermal Microdialysis Study
2.4.1. Animals
2.4.2. Surgery and Performance of Microdialysis Experiments
2.4.3. Sample Collection
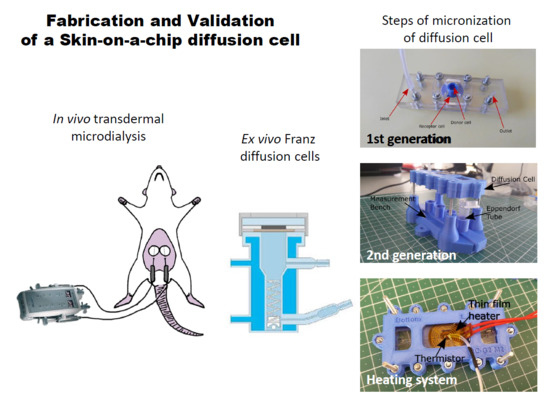
2.5. Skin-on-a-Chip Diffusion Cell Study
2.5.1. Design and Fabrication of the Device
2.5.2. Lab-on-a-Chip Sample Collection
2.6. Bioanalysis
2.6.1. Spectroscopy-Franz Diffusion Cell Samples
2.6.2. LC-MS/MS-Dialysate and Chip Samples
2.7. Histology
3. Results
3.1. Franz Diffusion Cell Studies
3.2. Transdermal Microdialysis Study
3.3. Skin-on-a-Chip Diffusion Cell Study
3.4. Histology
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leveque, N.; Makki, S.; Hadgraft, J.; Humbert, P. Comparison of Franz cells and microdialysis for assessing salicylic acid penetration through human skin. Int. J. Pharm. 2004, 269, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Iadaresta, F.; Manniello, M.D.; Östman, C.; Crescenzi, C.; Holmbäck, J.; Russo, P. Chemicals from textiles to skin: An in vitro permeation study of benzothiazole. Environ. Sci. Pollut. Res. Int. 2018, 25, 24629–24638. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Farner, F.; Bors, L.; Bajza, Á.; Karvaly, G.; Antal, I.; Erdő, F. Validation of an In vitro-in vivo Assay System for Evaluation of Transdermal Delivery of Caffeine. Drug Deliv. Lett. 2019, 9, 15–20. [Google Scholar] [CrossRef]
- Frosini, S.M.; Bond, R.; Loeffler, A.; Larner, J. Opportunities for topical antimicrobial therapy: Permeation of canine skin by fusidic acid. BMC Vet. Res. 2017, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.D.; de Groot, J.S. Percutaneous absorption of nafarelin acetate, an LHRH analog, through human cadaver skin and monkey skin. Int. J. Pharm. 1994, 110, 137–145. [Google Scholar] [CrossRef]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Mushroom ethanolic extracts as cosmeceuticals ingredients: Safety and ex vivo skin permeation studies. Food Chem. Toxicol. 2019, 127, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkó, S.; Zsikó, S.; Deák, G.; Gácsi, A.; Kovács, A.; Budai-Szűcs, M.; Pajor, L.; Bajory, Z.; Csányi, E. Papaverine hydrochloride containing nanostructured lyotropic liquid crystal formulation as a potential drug delivery system for the treatment of erectile dysfunction. Drug Des. Dev. Ther. 2018, 12, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Michniak-Kohn, B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Bal-Öztürk, A.; Miccoli, B.; Avci-Adali, M.; Mogtader, F.; Sharifi, F.; Çeçen, B.; Yaşayan, G.; Braeken, D.; Alarcin, E. Current Strategies and Future Perspectives of Skin-on-a-Chip Platforms: Innovations, Technical Challenges and Commercial Outlook. Curr. Pharm. Des. 2018, 24, 5437–5457. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F.A.; Eggert, S.; Wiest, J. Skin-on-a-Chip: Transepithelial Electrical Resistance and Extracellular Acidification Measurements through an Automated Air-Liquid Interface. Genes 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Dancik, Y.; Sriram, G.; Wu, B.; Teo, Y.L.; Feng, Z.; Bigliardi-Qi, M.; Wu, R.G.; Wang, Z.P.; Bigliardi, P.L. Multi-chamber microfluidic platform for high-precision skin permeation testing. Lab Chip 2017, 17, 1625–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Broek, L.J.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.H.; Heidary Araghi, B.; Beydaghi, V.; Geraili, A.; Moradi, F.; Jafari, P.; Janmaleki, M.; Valente, K.P.; Akbari, M.; Sanati-Nezhad, A. Skin Diseases Modeling using Combined Tissue Engineering and Microfluidic Technologies. Adv. Healthc. Mater. 2016, 5, 2459–2480. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F. Microdialysis Techniques in Pharmacokinetic and Biomarker Studies. Past, Present and Future Directions. A Review. Clin. Exp. Pharmacol. 2015, 5, 1459–2161. [Google Scholar] [CrossRef]
- Erdő, F.; Hashimoto, N.; Karvaly, G.; Nakamichi, N.; Kato, Y. Critical evaluation and methodological positioning of the transdermal microdialysis technique. A review. J. Control. Release 2016, 233, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Martínez, B.I.; Cazares-Delgadillo, J.; Calderilla-Fajardo, S.B.; Villalobos-García, R.; Ganem-Quintanar, A.; Quintanar-Guerrero, D. Preparation of polymeric nanocapsules containing octyl methoxycinnamate by the emulsification–diffusion technique: Penetration across the stratum corneum. J. Pharm. Sci. 2005, 94, 1552–1559. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The tape-stripping technique as a method for drug quantification in skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem Quintanar, A. Applications of the thermoreversible Pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Miyazaki, S.; Yokouchi, C.; Nakamura, T.; Hashiguchi, N.; Hou, W.M.; Takada, M. Pluronic F-127 gels as a novel vehicle for rectal administration of indomethacin. Chem. Pharm. Bull. 1986, 34, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.C.; Tan, H.K.; Chun, H.W. Antiinflammatory and Analgesic Transdermal Gel. U.S. Patent 5,527,832, 18 June 1996. [Google Scholar]
- Fang, J.Y.; Leu, Y.L.; Wang, Y.Y.; Tsai, Y.H. In vitro topical application and in vivo phramacodynamic evaluation of nonivamide hydrogels using Wistar rat as an animal model. Eur. J. Pharm. Sci. 2002, 15, 417–423. [Google Scholar] [CrossRef]
- Shin, S.C.; Cho, C.W.; Oh, I.J. Effects of non ionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. Int. J. Pharm. 2001, 222, 199–203. [Google Scholar] [CrossRef]
- Liaw, J.; Lin, Y.C. Evaluation of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) gels as a release vehicle for percutaneous fentanyl. J. Control. Release 2000, 68, 273–282. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hong, C.T.; Chiu, W.T.; Fang, J.Y. In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. Int. J. Pharm. 2001, 224, 89–104. [Google Scholar] [CrossRef]
- El-Kattan, A.F.; Asbill, C.S.; Kim, N.; Michniak, B.B. Effect of formulation variables on the percutaneous permeation of ketoprofen from gel formulations. Drug Deliv. 2000, 7, 147–153. [Google Scholar] [PubMed]
- Curdy, C.; Kalia, Y.N.; Naïk, A.; Guy, R.H. Piroxicam delivery into human stratum corneum in vivo: Iontophoresis versus passive diffusion. J. Control. Release 2001, 76, 73–79. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. In vivo skin permeation of sodium naproxen formulated in PF-127 gels: Effect of Azone® and Transcutol®. Drug Dev. Ind. Pharm. 2005, 31, 447–454. [Google Scholar] [CrossRef]
- Mattorano, D.A.; Kupper, L.L.; Nylander-French, L.A. Estimating dermal exposure to jet fuel (naphthalene) using adhesive tape strip samples. Ann. Occup. Hyg. 2004, 48, 139–146. [Google Scholar]
- Chao, Y.C.; Nylander-French, L.A. Determination of Keratin Protein in a Tape stripped Skin Sample from Jet Fuel Exposed Skin. Ann. Occup. Hyg. 2004, 48, 65–73. [Google Scholar]
- Surakka, J.; Lindh, T.; Rosen, G. Workers’ dermal exposure to UV-curable acrylates in the furniture and parquet industry. Ann. Occup. Hyg. 2000, 44, 635–644. [Google Scholar] [CrossRef]
- Nylander-French, L.A. A tape-stripping method for measuring dermal exposure to multifunctional acrylates. Ann. Occup. Hyg. 2000, 44, 645–651. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Pinkus, H. Tape stripping in dermatological research. A review with emphasis on epidermal biology. Giormale Ital. Derm. Minerva Dermatol. 1966, 107, 1115–1126. [Google Scholar]
- Löffler, H.; Dreher, F.; Maibach, H.I. Stratum corneum adhesive tape stripping: Influence of anatomical site, application pressure, duration and removal. Br. J. Dermatol. 2004, 151, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Mattern, K.; Beißner, N.; Reichl, S.; Dietzel, A. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 1—Engineering of microfluidic system and technical simulations. Eur. J. Pharm. Biopharm. 2018, 126, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Beiβner, N.; Mattern, K.; Dietzel, A.; Reichl, S. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 2—Ocular DynaMiTES for drug absorption studies of the anterior eye. Eur. J. Pharm. Biopharm. 2018, 126, 166–176. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Li, N.; Schwartz, M.; Ionescu-Zanetti, C. PDMS Compound Adsorption in Context. J. Biomol. Screen. 2009, 14, 194–202. [Google Scholar] [Green Version]
- Hönzke, S.; Wallmeyer, L.; Ostrowski, A.; Radbruch, M.; Mundhenk, L.; Schäfer-Korting, M.; Hedtrich, S. Influence of Th2 Cytokines on the Cornified Envelope, Tight Junction Proteins, and ß-Defensins in Filaggrin-Deficient Skin Equivalents. J. Investig. Dermatol. 2016, 136, 631–639. [Google Scholar] [CrossRef]
- Petrova, A.; Celli, A.; Jacquet, L.; Dafou, D.; Crumrine, D.; Hupe, M.; Arno, M.; Hobbs, C.; Cvoro, A.; Karagiannis, P.; et al. 3D in vitro model of a functional epidermal permeability barrier from human embryonic stem cells and induced pluripotent stem cells. Stem. Cell Rep. 2014, 2, 675–689. [Google Scholar] [CrossRef] [PubMed]
- van Drongelen, V.; Danso, M.O.; Mulder, A.; Mieremet, A.; van Smeden, J.; Bouwstra, J.A.; El Ghalbzouri, A. Barrier properties of an N/TERT-based human skin equivalent. Tissue Eng. Part A 2014, 20, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Kostka, K.H.; Lehr, C.M.; Schaefer, U.F. Interrelation of permeation and penetration parameters obtained from in vitro experiments with human skin and skin equivalents. J. Control. Release 2001, 75, 283–295. [Google Scholar] [CrossRef]
- Zghoul, N.; Fuchs, R.; Lehr, C.M.; Schaefer, U.F. Reconstructed skin equivalents for assessing percutaneous drug absorption from pharmaceutical formulations. Altex 2001, 18, 103–106. [Google Scholar] [PubMed]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs—Design, Fabrication, and Testing. Pharmaceutics 2019, 11, 445. https://doi.org/10.3390/pharmaceutics11090445
Lukács B, Bajza Á, Kocsis D, Csorba A, Antal I, Iván K, Laki AJ, Erdő F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs—Design, Fabrication, and Testing. Pharmaceutics. 2019; 11(9):445. https://doi.org/10.3390/pharmaceutics11090445
Chicago/Turabian StyleLukács, Bence, Ágnes Bajza, Dorottya Kocsis, Attila Csorba, István Antal, Kristóf Iván, András József Laki, and Franciska Erdő. 2019. "Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs—Design, Fabrication, and Testing" Pharmaceutics 11, no. 9: 445. https://doi.org/10.3390/pharmaceutics11090445