A Prospective Study of Different Types of Dietary Fiber and Risk of Cardiovascular Disease: Tehran Lipid and Glucose Study
Abstract
:1. Introduction
2. Methods
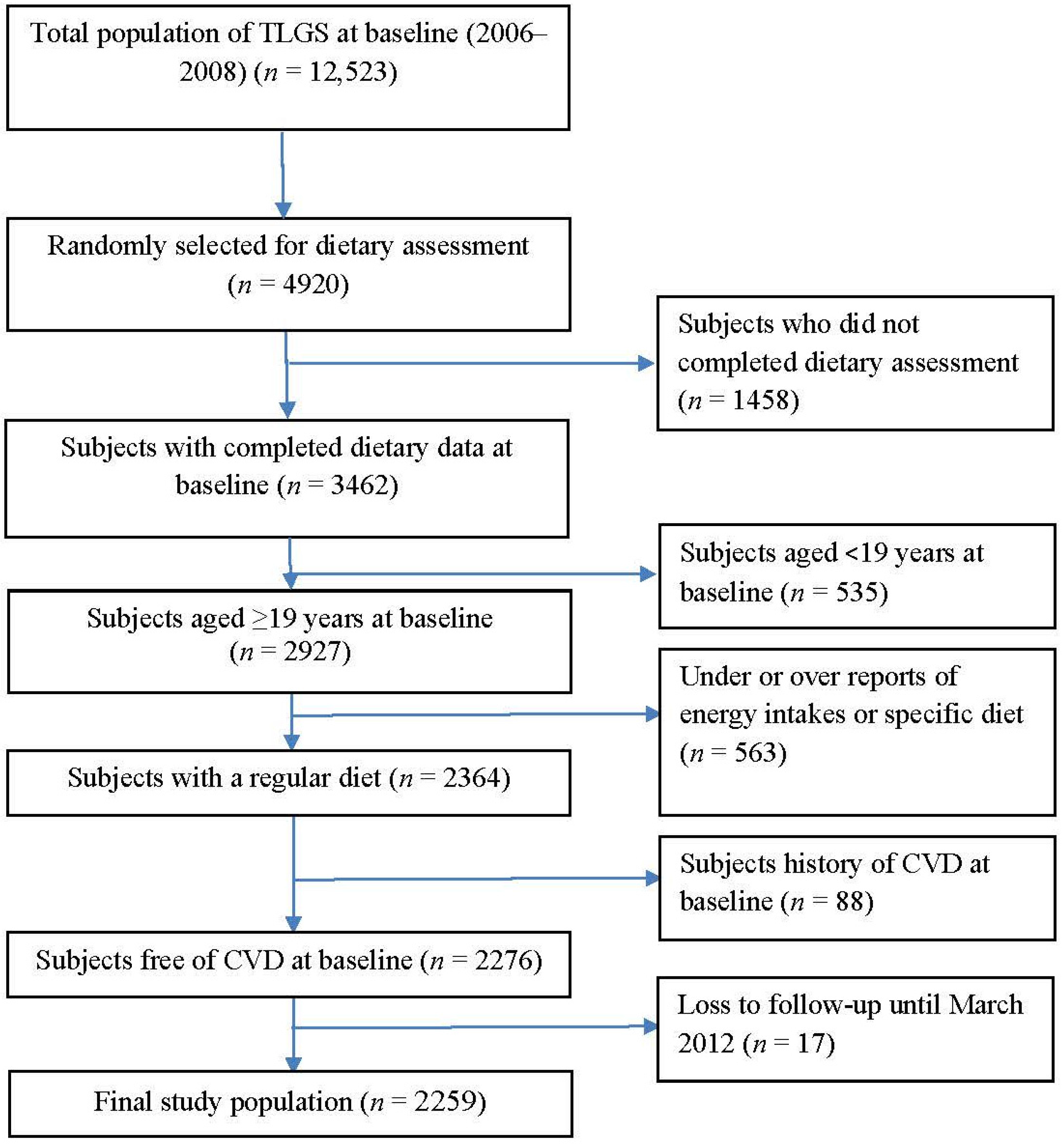
2.1. Study Population
2.2. Demographic and Anthropometric Measures
2.3. Biochemical Measures
2.4. Dietary Assessment
2.5. Definition of Terms
2.6. Definition of Outcome in Our Study
2.7. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pereira, M.A.; Liu, S. Types of carbohydrates and risk of cardiovascular disease. J. Womens Health 2003, 12, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef] [PubMed]
- Erkkila, A.T.; Lichtenstein, A.H. Fiber and cardiovascular disease risk: How strong is the evidence? J. Cardiovasc. Nurs. 2006, 21, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Eshak, E.S.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Wakai, K.; Tamakoshi, A.; JACC Study Group. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J. Nutr. 2010, 140, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Iso, H.; Saito, I.; Yamagishi, K.; Ishihara, J.; Inoue, M.; Tsugane, S.; JPHC Study Group. Dietary fiber intake and risk of cardiovascular disease in the Japanese population: The Japan Public Health Center-based study cohort. Eur. J. Clin. Nutr. 2011, 65, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.M.P.; Sohrab, G.; Hosseini-Esfahani, F.; Azizi, F. Inverse association between fruit, legume, and cereal fiber and the risk of metabolic syndrome: Tehran Lipid and Glucose Study. Diabetes Res. Clin. Pract. 2011, 94, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [PubMed]
- Liu, S.; Buring, J.E.; Sesso, H.D.; Rimm, E.B.; Willett, W.C.; Manson, J.E. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J. Am. Coll. Cardiol. 2002, 39, 49–56. [Google Scholar] [CrossRef]
- Azizi, F.; Rahmani, M.; Emami, H.; Mirmiran, P.; Hajipour, R.; Madjid, M.; Ghanbili, J.; Ghanbarian, A.; Mehrabi, Y.; Saadat, N.; et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1). Soz. Praventivmed. 2002, 47, 408–426. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Esfahani, F.; Jessri, M.; Mirmiran, P.; Bastan, S.; Azizi, F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metab. Clin. Exp. 2010, 59, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Esfahani, F.H.; Mehrabi, Y.; Hedayati, M.; Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010, 13, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Skoumas, Y.; Stefanadis, C. The Association between Food Patterns and the Metabolic Syndrome Using Principal Components Analysis: The ATTICA Study. J. Am. Diet. Assoc. 2007, 107, 979–987. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Borai, A.; Livingstone, C.; Kaddam, I.; Ferns, G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med. Res. Methodol. 2011, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 94, E15–E26. [Google Scholar] [CrossRef] [PubMed]
- Enzevaei, A.; Salehpour, S.; Tohidi, M.; Saharkhiz, N. Subclinical hypothyroidism and insulin resistance in polycystic ovary syndrome: Is there a relationship? Iran. J. Reprod. Med. 2014, 12, 481–486. [Google Scholar] [PubMed]
- Hadaegh, F.; Harati, H.; Ghanbarian, A.; Azizi, F. Association of total cholesterol versus other serum lipid parameters with the short-term prediction of cardiovascular outcomes: Tehran Lipid and Glucose Study. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Nejat, A.; Mirbolouk, M.; Mohebi, R.; Hasheminia, M.; Tohidi, M.; Saadat, N.; Azizi, F.; Hadaegh, F. Changes in lipid measures and incident coronary heart disease: Tehran Lipid & Glucose Study. Clin. Biochem. 2014, 47, 1239–1244. [Google Scholar] [PubMed]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar] [PubMed]
- Rimm, E.B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996, 275, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Kumanyika, S.K.; Lemaitre, R.N.; Olson, J.L.; Burke, G.L.; Siscovick, D.S. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA 2003, 289, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 years: The Zutphen Study. Am. J. Clin. Nutr. 2008, 88, 1119–1125. [Google Scholar] [PubMed]
- Satija, A.; Hu, F.B. Cardiovascular benefits of dietary fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Aljuraiban, G.S.; Griep, L.M.; Chan, Q.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S. Total, insoluble and soluble dietary fibre intake in relation to blood pressure: The INTERMAP Study. Br. J. Nutr. 2015, 114, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Van Horn, L.; Shay, C.M.; Lloyd-Jones, D.M. Associations of Dietary Fiber Intake with Long-Term Predicted Cardiovascular Disease Risk and C-Reactive Protein Levels (from the National Health and Nutrition Examination Survey Data. Am. J. Cardiol. 2014, 113, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Doostvandi, T.; Bahadoran, Z.; Mozaffari-Khosravi, H.; Mirmiran, P.; Azizi, F. Food intake patterns are associated with the risk of impaired glucose and insulin homeostasis: A prospective approach in the Tehran Lipid and Glucose Study. Public Health Nutr. 2016, 19, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Hosseini-Esfahani, F.; Hosseinpanah, F.; Mirmiran, P.; Azizi, F. Patterns of food consumption and risk of type 2 diabetes in an Iranian population: A nested case–control study. Nutr. Diet. 2016, 73, 169–176. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic Effects of Dietary Fiber Consumption and Prevention of Diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [PubMed]
- Anderson, J.W.; Tietyen-Clark, J. Dietary fiber: Hyperlipidemia, hypertension, and coronary heart disease. Am. J. Gastroenterol. 1986, 81, 907–919. [Google Scholar] [PubMed]
- Anderson, J.W.; Chen, W.J. Plant fiber. Carbohydrate and lipid metabolism. Am. J. Clin. Nutr. 1979, 32, 346–363. [Google Scholar] [PubMed]
- Kuo, S.M. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr. 2013, 4, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Giorgio, V.; Alberelli, M.A.; De Candia, E.; Gasbarrini, A.; Grieco, A. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2015, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef] [PubMed]

| Participants with CVD Outcome (n = 57) | Participants without CVD Outcome (n = 2202) | p Value | |
|---|---|---|---|
| Age (years) | 58.6 ± 9.7 | 37.3 ± 13.0 | 0.001 |
| Male (%) | 59.6 | 42.5 | 0.001 |
| Smoking (%) | 26.3 | 22.7 | 0.31 |
| Body mass index (m2/kg) | 28.4 ± 4.4 | 26.5 ± 4.8 | 0.005 |
| Lipid-lowering drugs (%) | 10.5 | 1.5 | 0.001 |
| Anti-hypertensive drugs (%) | 14.3 | 1.5 | 0.001 |
| Aspirin (%) | 8.9 | 2.3 | 0.001 |
| Waist circumference (cm) | 97.2 ± 10.1 | 88.0 ± 13.2 | 0.001 |
| Serum creatinine (μmol/L) | 1.13 ± 0.18 | 1.03 ± 0.15 | 0.001 |
| Systolic blood pressure (mmHg) | 130 ± 17.4 | 109 ± 14.9 | 0.001 |
| Diastolic blood pressure (mmHg) | 80.2 ± 10.6 | 72.5 ± 10.3 | 0.001 |
| Fasting blood glucose (mg/dL) | 106 ± 39.9 | 88.5 ± 16.7 | 0.001 |
| Serum triglycerides (mg/dL) | 190 ± 107 | 132 ± 78.0 | 0.001 |
| HDL-C (mg/dL) | 40.2 ± 8.0 | 43.3 ± 10.4 | 0.03 |
| TG/HDL-ratio | 5.0 ± 3.2 | 3.4 ± 2.7 | 0.001 |
| Serum insulin | 7.4 ± 3.4 | 8.8 ± 4.9 | 0.13 |
| HOMA-IR | 1.5 ± 0.8 | 1.9 ± 0.8 | 0.28 |
| Hyperinsulinemia (%) | 12.5 | 17.6 | 0.57 |
| Diabetes (%) | 14.3 | 3.9 | 0.002 |
| Hypertension (%) | 43.9 | 8.3 | 0.001 |
| Cardiovascular disease risk score | 22.1 | 21.2 | 0.001 |
| Dietary intakes | |||
| Total fats (g/day) | 74.1 ± 2.5 | 79.5 ± 0.4 | 0.03 |
| Saturated fats (g/day) | 24.8 ± 1.8 | 27.1 ± 0.3 | 0.22 |
| Mono-unsaturated fat (g/day) | 21.9 ± 1.0 | 27.6 ± 0.2 | 0.01 |
| Sodium (mg/day) | 4883 ± 410 | 4421 ± 66 | 0.26 |
| Total carbohydrate (g/day) | 337 ± 5.8 | 323 ± 0.9 | 0.02 |
| Total fiber (g/day) | 25.3 ± 1.1 | 29.0 ± 0.2 | 0.003 |
| Dietary Fiber Intakes | T1 | T2 | T3 | p for Trend |
|---|---|---|---|---|
| Total fiber | ||||
| Crude | Ref. | 0.83 (0.45–1.54) | 0.69 (0.36–1.33) | 0.56 |
| Model 1 | Ref. | 0.87 (0.46–1.62) | 0.75 (0.38–1.46) | 0.69 |
| Model 2 | Ref. | 0.67 (0.35–1.26) | 0.39 (0.18–0.83) | 0.05 |
| Soluble fiber | ||||
| Crude | Ref. | 0.49 (0.26–0.91) | 0.39 (0.20–0.76) | 0.01 |
| Model 1 | Ref. | 0.54 (0.29–1.03) | 0.41 (0.20–0.82) | 0.04 |
| Model 2 a | Ref. | 0.39 (0.21–0.75) | 0.19 (0.09–0.41) | 0.001 |
| Insoluble fiber | ||||
| Crude | Ref. | 0.64 (0.34–1.18) | 0.53 (0.28–1.02) | 0.15 |
| Model 1 | Ref. | 0.72 (0.39–1.33) | 0.58 (0.29–1.14) | 0.25 |
| Model 2 b | Ref. | 0.54 (0.28–1.03) | 0.31 (0.14–0.69) | 0.014 |
| Dietary Fibers | T1 | T2 | T3 | p for Trend |
|---|---|---|---|---|
| Grain fiber | ||||
| Crude | Ref. | 0.89 (0.46–1.72) | 1.11 (0.59–1.07) | 0.79 |
| Model 1 | Ref. | 0.83 (0.42–1.62) | 0.98 (0.52–1.84) | 0.84 |
| Model 2 | Ref. | 0.79 (0.39–1.61) | 0.90 (0.44–1.86) | 0.82 |
| Legume fiber | ||||
| Crude | Ref. | 0.61 (0.34–1.11) | 0.36 (0.18–0.73) | 0.01 |
| Model 1 | Ref. | 0.59 (0.32–1.09) | 0.38 (0.18–0.77) | 0.02 |
| Model 2 | Ref. | 0.47 (0.25–0.89) | 0.31 (0.15–0.65) | 0.003 |
| Nut fiber | ||||
| Crude | Ref. | 0.78 (0.43–1.41) | 0.47 (0.24–0.94) | 0.10 |
| Model 1 | Ref. | 0.77 (0.43–1.42) | 0.54 (0.27–1.07) | 0.21 |
| Model 2 | Ref. | 0.65 (0.33–1.27) | 0.49 (0.24–1.02) | 0.14 |
| Fruit fiber | ||||
| Crude | Ref. | 0.74 (0.39–1.39) | 0.81 (0.43–1.50) | 0.61 |
| Model 1 | Ref. | 0.74 (0.38–1.41) | 0.83 (0.44–1.58) | 0.65 |
| Model 2 | Ref. | 0.56 (0.29–1.09) | 0.44 (0.22–0.89) | 0.05 |
| Vegetable fiber | ||||
| Crude | Ref. | 0.83 (0.45–1.52) | 0.59 (0.31–1.16) | 0.32 |
| Model 1 | Ref. | 0.82 (0.45–1.51) | 0.61 (0.31–1.21) | 0.37 |
| Model 2 | Ref. | 0.64 (0.34–1.20) | 0.34 (0.16–0.72) | 0.02 |
| Author | Study Population | Findings |
|---|---|---|
| Rimm et al. [25] | 6-year follow-up study among adult men | Dietary intake of fiber 28.9 vs. 12.4 g/day decreased risk of total myocardial infarction (RR = 0.59, 95% CI = 0.46–0.76) and fatal myocardial infarction RR = 0.45, 95% CI = 0.28–0.72. Cereal fiber reduced risk of total MI (RR = 0.71, 95% CI = 0.55–0.91 for each 10 g/day increase in cereal fiber) |
| Mozaffarian et al. [26] | 8.6-year follow-up of elderly men and women | Highest compared to the lowest quintile of cereal fiber consumption, decreased incident CVD (HR = 0.79; 95% CI = 0.62–0.99) Fruit fiber and vegetable fiber intake were not associated with incident CVD. Higher intake of cereal fiber was associated with lower risk of total stroke, ischemic stroke and ischemic heart disease death. |
| Streppel et al. [28] | 40-year follow-up of adult men | Every additional 10 g/day of dietary fiber intake decreased CVD mortality by 17% (95% CI: 2%, 30%) and all-cause mortality by 9% (0%, 18%). |
| Threapleton et al. [27] | Meta-analysis of 22 prospective cohorts | Total fiber intake was inversely associated with risk of CVD (RR = 0.91 per 7 g/day, 95% CI = 0.88–0.94) and CVD (RR = 0.91, 95% CI = 0.87–0.94). Each 7 g/day increase in insoluble fiber (RR = 0.82, 95% CI = 0.70–0.96), fiber from cereal (RR = 0.84, 95% CI = 0.76 0.94), and each 4 g/day increase in fiber from vegetable sources (RR = 0.94, 95% CI = 0.89–1.00) decreased risk of CVD and CVD. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirmiran, P.; Bahadoran, Z.; Khalili Moghadam, S.; Zadeh Vakili, A.; Azizi, F. A Prospective Study of Different Types of Dietary Fiber and Risk of Cardiovascular Disease: Tehran Lipid and Glucose Study. Nutrients 2016, 8, 686. https://doi.org/10.3390/nu8110686
Mirmiran P, Bahadoran Z, Khalili Moghadam S, Zadeh Vakili A, Azizi F. A Prospective Study of Different Types of Dietary Fiber and Risk of Cardiovascular Disease: Tehran Lipid and Glucose Study. Nutrients. 2016; 8(11):686. https://doi.org/10.3390/nu8110686
Chicago/Turabian StyleMirmiran, Parvin, Zahra Bahadoran, Sajad Khalili Moghadam, Azita Zadeh Vakili, and Fereidoun Azizi. 2016. "A Prospective Study of Different Types of Dietary Fiber and Risk of Cardiovascular Disease: Tehran Lipid and Glucose Study" Nutrients 8, no. 11: 686. https://doi.org/10.3390/nu8110686




