The Effect of a 20 km Run on Appetite Regulation in Long Distance Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Blood Parameters
2.4. Subjective Feelings of Hunger, Appetite, Perceived Food Consumption, Satiety, and Fatigue
2.5. Respiratory Parameters
2.6. Ad Libitum Buffet Meal
2.7. Statistical Analysis
3. Results
3.1. Exercise Duration and Heart Rate Response during the 20 km Run
3.2. Scores for Subjective Appetite and Fatigue
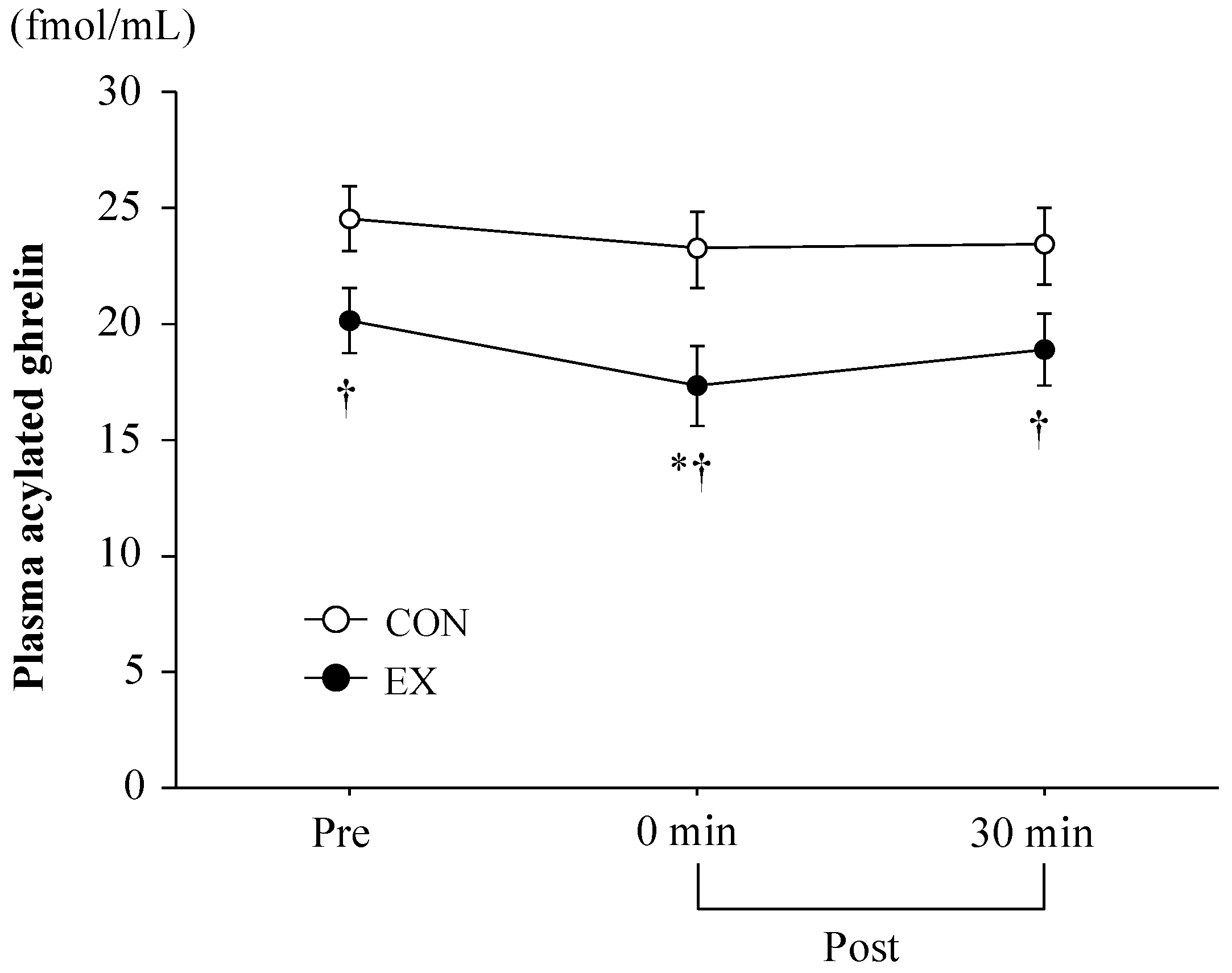
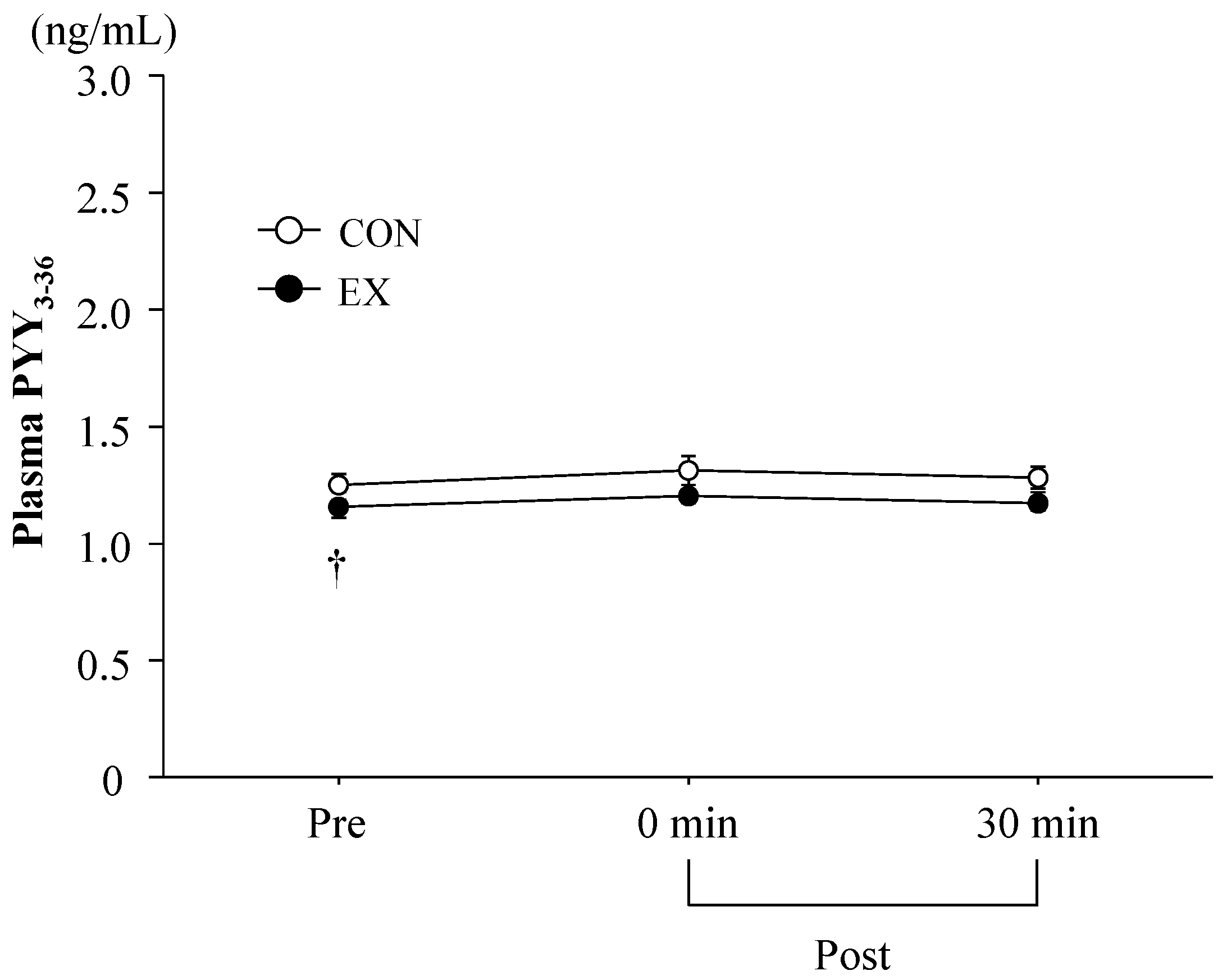
3.3. Blood Parameters
3.4. Respiratory Parameters
3.5. Energy and Macronutrient Intake
3.6. Inter-Individual Variability in Exercise-Induced Changes in Energy Intake
3.7. Correlation between Exercise-Induced Relative Changes in Energy Intake and Blood Variables
4. Discussion
4.1. Exercise-Induced Ghrelin and PYY3-36 Responses
4.2. Energy Intake Following 20 km Run
4.3. Inter-Individual Variability of Exercise-Induced Reduction of Energy Intake
4.4. Macronutrient Intake Following a 20 km Run
4.5. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PYY | peptide YY |
| GLP-1 | glucagon-like peptide-1 |
| O2max | maximal oxygen uptake |
| HIIT | high-intensity interval training |
| VAS | visual analog scale |
| GH | growth hormone |
| FFA | free fatty acid |
| Mb | myoglobin |
| CK | creatine kinase |
| CV | coefficients of variation |
| IL-6 | interleukin-6 |
References
- Kojima, M.; Kanagawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Broom, S.R. The gut hormone peptide YY regulates appetite. Ann. N. Y. Acad. Sci. 2003, 994, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Battrerham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, B.; Williams, G.; Ghatei, M.A.; Bloom, S.R. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987, 2, 1300–1304. [Google Scholar] [CrossRef]
- King, J.A.; Miyashita, M.; Wasse, L.K.; Stensel, D.J. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite 2010, 54, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effect of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Jokisch, E.; Coletta, A.; Raynor, H.A. Acute energy compensation and macronutrient intake following exercise in active and inactive males who are normal weight. Appetite 2012, 58, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; King, N.A.; Blundell, J.E. Uncoupling the effects of energy expenditure and energy intake: Appetite pesponse to short-term energy deficit induced by meal omission and physical activity. Appetite 1998, 31, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pomerleau, M.; Imbeault, P.; Parker, T.; Doucet, E. Effects of exercise intensity on food intake and appetite in women. Am. J. Clin. Nutr. 2004, 80, 1230–1236. [Google Scholar] [PubMed]
- Broom, D.R.; Batterham, R.L.; King, J.A.; Stensel, D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 269, R29–R35. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.K.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int. J. Obes. (Lond.) 2014, 38, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake response to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Levenhagen, D.K.; Gresham, J.D.; Carlson, M.G.; Maron, D.J.; Borel, M.J.; Flakoll, O.J. Postexersie nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E982–E993. [Google Scholar] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Bruns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Mineta, M.; Asaka, M.; Miyashita, M.; Numao, S.; Gando, Y.; Ando, T.; Sakamoto, S.; Higuchi, M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite 2013, 66, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, T.; Ueno, H.; Toshinai, K.; Kawagoe, T.; Naito, S.; Tobina, T.; Nishida, Y.; Shindo, M.; Kangawa, K.; Tanaka, H.; et al. Significant lowering of plasma ghrelin but not des-acyl ghrelin in response to acute exercise in men. Endocr. J. 2011, 58, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Dall, R.; Kanaley, J.; Hansen, T.K.; Møller, N.; Christiansen, J.S.; Hosoda, H.; Kangawa, K.; Jørgensen, J.O. Plasma ghrelin levels during exercise in healthy subjects and in growth hormone-deficient patients. Eur. J. Endocrinol. 2002, 147, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, M.; Kordi, M.R.; Taghikhani, M.; Ravasi, A.A.; Gaeini, A.A.; Tabrizi, A. Possible role for growth hormone in suppressing acylated ghrelin and hunger ratings during and after intermittent exercise of different intensities in obese individuals. Acta Med. Iran. 2014, 52, 29–37. [Google Scholar] [PubMed]
- Vestergaad, E.T.; Dall, R.; Lange, K.H.; Kjaer, M.; Christiansen, J.S.; Jorgensen, J.O. The ghrelin responses to exercise before and after growth hormone administration. J. Clin. Endocrinol. Metab. 2007, 92, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.A.; Yamashiro, M.; Hinkel-Lipsker, J.; Streder, K.; Evero, N.; Hackney, T. Effects of acute exercise on appetite hormones and ad libitum energy intake in men and women. Appl. Physiol. Nutr. Metab. 2013, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Stensel, D.J. Creating an acute energy intake deficient without stimulating compensatory increases in appetite: Is there an optimal exercise protocol? Proc. Nutr. Soc. 2014, 73, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bryant, E.; Blundell, J.E.; King, N.A. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol. Behav. 2009, 97, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Blundel, J.E.; King, N.A. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br. J. Sports Med. 2014, 48, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; Otto, A.D.; Goodpaster, B.H.; Helsel, D.L.; Pellegrini, C.A.; Jakicic, J.M. Acute effects of walking on energy intake in overweight/obese women. Appetite 2010, 55, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Eliingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhance insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cell. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2009, 12, 6–33. [Google Scholar]
- Shorten, A.L.; Wallman, K.E.; Guelfi, K.J. Acute effect of environment temperature during exercise on subsequent energy in active men. Am. J. Clin. Nutr. 2009, 90, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.J.; Roberts, S.B. The effects of exercise on food intake and body fatness: A summary of published studies. Nutr. Rev. 2007, 65, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-plantenga, M.S.; Verwegen, C.R.; Ijedema, M.J.; Wijckmans, N.E.; Saris, W.H. Acute effects of exercise or sauna on appetite in obese and nonobese men. Physiol. Behav. 1997, 62, 1345–1354. [Google Scholar] [CrossRef]
- Blundell, J.E.; Stubbs, R.J.; Hughes, D.A.; Whybrow, S.; King, N.A. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc. Nutr. Soc. 2003, 62, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; O’Leary, K.C.; Dorfman, L.; Thomas, J.G.; Strohacker, K.; Wing, R.R. Consistency in compensatory eating responses following acute exercise in inactive, overweight and obese women. Br. J. Nutr. 2015, 113, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, K.; Olver, T.D.; Abbott, K.C.; Lemon, P.W. Energy intake over 2 days is unaffected by acute sprint interval exercise despite increased appetite and energy expenditure. Appl. Physiol. Nutr. Metab. 2014, 40, 79–86. [Google Scholar] [CrossRef] [PubMed]

| Pre | Post | After Meal | ||||
|---|---|---|---|---|---|---|
| 0 min | 15 min | 30 min | ||||
| Hunger (mm) | EX | 57 ± 4 | 51 ± 6 † | 60 ± 5 † | 68 ± 4 | 11 ± 1 * |
| CON | 60 ± 3 | 70 ± 3 * | 71 ± 3 * | 72 ± 3 * | 16 ± 3 * | |
| Appetite (mm) | EX | 59 ± 5 | 52 ± 7 † | 62 ± 5 † | 68 ± 5 | 18 ± 4 * |
| CON | 58 ± 4 | 70 ± 3 * | 72 ± 3 * | 72 ± 3 * | 23 ± 4 * | |
| Prospective food consumption (mm) | EX | 63 ± 4 | 54 ± 6 † | 61 ± 5 | 69 ± 4 * | 20 ± 4 * |
| CON | 58 ± 4 | 67 ± 3 * | 70 ± 2 * | 68 ± 3 | 22 ±3 * | |
| Satiety (mm) | EX | 30 ± 4 | 29 ± 5 † | 32 ± 5 † | 31 ± 5 † | 82 ± 4 *,† |
| CON | 26 ± 3 | 20 ± 3 | 18 ± 5 | 18 ± 3 | 70 ± 5 * | |
| Fatigue (mm) | EX | 43 ± 4 † | 58 ± 4 *,† | 55 ± 4 *,† | 52 ± 4 † | 41 ± 4 † |
| CON | 29 ± 3 | 26 ± 3 | 23 ± 3 * | 24 ± 4 | 26 ± 4 | |
| Pre | Post | |||
|---|---|---|---|---|
| 0 min | 30 min | |||
| Glucose (mmol/L) | EX | 4.92 ± 0.05 † | 5.30 ± 0.12 *,† | 4.64 ± 0.08 *,† |
| CON | 4.78 ± 0.05 | 4.84 ± 0.05 | 4.93 ± 0.04 * | |
| Lactate (mmol/L) | EX | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.1 |
| CON | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | |
| GH (ng/mL) | EX | 1.8 ± 0.5 | 8.9 ± 1.8 *,† | 4.1 ± 0.8 † |
| CON | 2.5 ± 0.6 | 2.1 ± 0.4 | 1.3 ± 0.3 | |
| FFA (mmol/L) | EX | 0.42 ± 0.05 | 1.22 ± 0.08 *,† | 0.90 ± 0.08 *,† |
| CON | 0.38 ± 0.03 | 0.35 ± 0.03 | 0.55 ± 0.04 * | |
| Mb (ng/mL) | EX | 36 ± 2 | 136 ± 26 *,† | 140 ± 22 *,† |
| CON | 37 ± 3 | 36 ± 2 | 35 ± 2 | |
| CK | EX | 349 ± 27 | 457 ± 30 * | 436 ± 29 * |
| (U/L) | CON | 402 ± 74 | 389 ± 68 * | 385 ± 70 * |
| EX | CON | ||
|---|---|---|---|
| General information | |||
| Duration of eating | (min) | 22 ± 1 | 23 ± 1 |
| Energy intake | (kcal) | 1325 ± 55 † | 1529 ± 55 |
| Detailed information | |||
| Macronutrient intake | |||
| Protein | (%) | 14.6 ± 0.5 | 15.3 ± 0.5 |
| (g) | 49 ± 3 † | 58 ± 2 | |
| Fat | (%) | 26.2 ± 1.4 † | 29.8 ± 1.2 |
| (g) | 39 ± 3 | 51 ± 3 | |
| Carbohydrate | (%) | 59.2 ± 1.9 † | 54.9 ± 1.5 |
| (g) | 190 ± 9 | 202 ± 10 | |
| Categories of selected menus | |||
| Staple food (rice and bread) | (kcal) | 545 ± 34 † | 659 ± 45 |
| Others | (kcal) | 553 ± 38 † | 694 ± 28 |
| Fruits | (kcal) | 101 ± 15 | 75 ± 12 |
| Drinks (tea, juice milk and soup) | (kcal) | 126 ± 11 † | 101 ± 16 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, C.; Ishibashi, A.; Ebi, K.; Goto, K. The Effect of a 20 km Run on Appetite Regulation in Long Distance Runners. Nutrients 2016, 8, 672. https://doi.org/10.3390/nu8110672
Kojima C, Ishibashi A, Ebi K, Goto K. The Effect of a 20 km Run on Appetite Regulation in Long Distance Runners. Nutrients. 2016; 8(11):672. https://doi.org/10.3390/nu8110672
Chicago/Turabian StyleKojima, Chihiro, Aya Ishibashi, Kumiko Ebi, and Kazushige Goto. 2016. "The Effect of a 20 km Run on Appetite Regulation in Long Distance Runners" Nutrients 8, no. 11: 672. https://doi.org/10.3390/nu8110672





