The Relationship between Metabolic Syndrome and Osteoporosis: A Review
Abstract
:1. Introduction
2. The Association between MetS and Osteoporosis
2.1. In Vivo Studies
2.1.1. Obesity
2.1.2. Dyslipidemia
2.1.3. Hyperglycemia
2.1.4. Hypertension
2.2. Human Studies
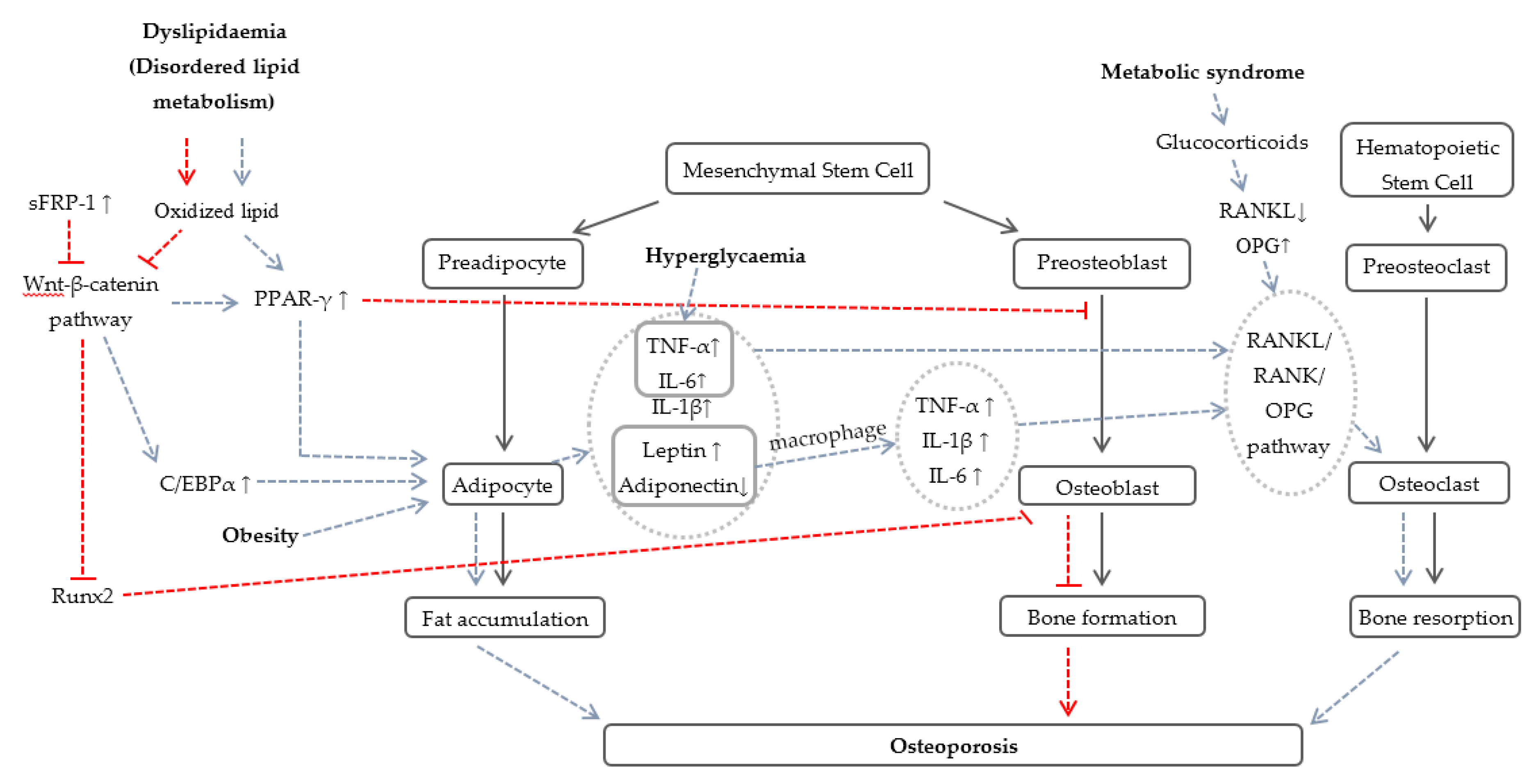
2.3. Potential Mechanisms Involved in Osteoporosis Due to Components of MetS
2.4. Relationship between Gluco-Mineral-Corticoids, MetS, and Osteoporosis
3. Therapeutic Treatment for MetS and Osteoporosis
4. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| BMC | bone mineral content |
| BMD | bone mineral density |
| BMI | body mass index |
| BV/TV | bone volume/total volume |
| C/EBPα | CCAAT-enhancer binding protein α |
| CTX | C-terminal telopeptide of type-1 collagen |
| DEXA | dual-energy X-ray absorptiometry |
| FN-BMD | femoral neck bone mineral density |
| HDL | high density lipoprotein |
| HF | high-fat |
| HFHC | high-fat high-cholesterol |
| IGF-1 | insulin-like growth factor-1 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| LDL | low density lipoprotein |
| MetS | metabolic syndrome |
| NaCl | sodium chloride |
| OPG | osteoprotegerin |
| PINP | amino-terminal propeptide of type-1 collagen |
| PPAR-γ | peroxisome proliferator-activated receptor-γ |
| PTH | parathyroid hormone |
| RANK | receptor activator of NF-κB |
| RANKL | receptor activator of NF-κB ligand |
| Runx2 | runt-related transcription factor-2 |
| sFRP-1 | secreted frizzled-related protein 1 |
| SHR | spontaneous hypertensive rats |
| STZ | streptozotocin |
| Tb.N | trabecular number |
| Tb.Sp | trabecular separation |
| Tb.Th | trabecular thickness |
| TNF-α | tumor necrosis factor-α |
| WKY | Wistar-Kyoto rats |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.; Lyu, R.; Low, L.P.; Sheu, W.H.; Nitiyanant, W.; Saito, I.; Tan, C.E. Metabolic syndrome: Recent prevalence in East and Southeast Asian populations. Asia Pac. J. Clin. Nutr. 2007, 16, 362–367. [Google Scholar] [PubMed]
- Pan, W.H.; Yeh, W.T.; Weng, L.C. Epidemiology of metabolic syndrome in Asia. Asia Pac. J. Clin. Nutr. 2008, 17, 37–42. [Google Scholar] [PubMed]
- Sugimoto, T.; Sato, M.; Dehle, F.C.; Brnabic, A.J.; Weston, A.; Burge, R. Lifestyle-related metabolic disorders, osteoporosis, and fracture risk in Asia: A systematic review. Value Health Reg. Issues 2016, 9, 49–56. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Osteoporosis at the Primary Health Care Level: Summary Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Nayak, N.K.; Khedkar, C.C.; Khedkar, G.D.; Khedkar, C.D. Osteoporosis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 181–185. [Google Scholar]
- Hippisley-Cox, J.; Coupland, C. Predicting risk of osteoporotic fracture in men and women in England and Wales: Prospective derivation and validation of QFractureScores. BMJ 2009, 339, b4229. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Trajanoska, K.; Kiefte-de Jong, J.C.; Oei, L.; Uitterlinden, A.G.; Hofman, A.; Dehghan, A.; Zillikens, M.C.; Franco, O.H.; Rivadeneira, F. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: The Rotterdam Study. PLoS ONE 2015, 10, e0129116. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, R.; Albertse, E.C.; Hough, S. Body fat distribution as a risk factor for osteoporosis. S. Afr. Med. J. 1996, 86, 1081–1084. [Google Scholar] [PubMed]
- Jankowska, E.A.; Rogucka, E.; Medras, M. Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia 2001, 33, 384–389. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Oden, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Von Muhlen, D.; Safii, S.; Jassal, S.K.; Svartberg, J.; Barrett-Connor, E. Associations between the metabolic syndrome and bone health in older men and women: The Rancho Bernardo Study. Osteoporos. Int. 2007, 18, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Braga, V.; Zamboni, M.; Gatti, D.; Rossini, M.; Bakri, J.; Battaglia, E. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif. Tissue Int. 2004, 74, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sugimoto, T.; Yano, S.; Yamauchi, M.; Sowa, H.; Chen, Q.; Chihara, K. Plasma lipids and osteoporosis in postmenopausal women. Endocr. J. 2002, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Kritz-Silverstein, D. Does hyperinsulinemia preserve bone? Diabetes Care 1996, 19, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Nishio, I.; Masuyama, Y. Bone mineral density in women with essential hypertension. Am. J. Hypertens. 2001, 14, 704–707. [Google Scholar] [CrossRef]
- Hanley, D.A.; Brown, J.P.; Tenenhouse, A.; Olszynski, W.P.; Ioannidis, G.; Berger, C.; Prior, J.C.; Pickard, L.; Murray, T.M.; Anastassiades, T.; et al. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: Cross-sectional results from the Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2003, 18, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, V.; Nandhini, A.T.A.; Anuradha, C.V. Lipoic acid attenuates hypertension and improves insulin sensitivity, kallikrein activity and nitrite levels in high fructose-fed rats. J. Comp. Physiol. B 2004, 174, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Oron-Herman, M.; Kamari, Y.; Grossman, E.; Yeger, G.; Peleg, E.; Shabtay, Z.; Shamiss, A.; Sharabi, Y. Metabolic syndrome: Comparison of the two commonly used animal models. Am. J. Hypertens. 2008, 21, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Yusof, M.R.M.; Suhaimi, F.H. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, J.; Zhang, W.; Zhuang, X.; Wang, J.; Xu, R.; Xu, Z.; Qu, W. Antihypertensive effect of total flavones extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J. Ethnopharmacol. 2008, 117, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.P.; Coppey, L.J.; Dake, B.; Yorek, M.A. Effect of treatment of sprague dawley rats with AVE7688, enalapril, or candoxatril on diet-induced obesity. J. Obes. 2011, 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.; Brown, L. Comparison of purple carrot juice and beta-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Dissard, R.; Klein, J.; Caubet, C.; Breuil, B.; Siwy, J.; Hoffman, J.; Sicard, L.; Ducassé, L.; Rascalou, S.; Payre, B.; et al. Long term metabolic syndrome induced by a high fat high fructose diet leads to minimal renal injury in C57BL/6 mice. PLoS ONE 2013, 8, e76703. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Narukawa, M.; Watanabe, T. Adiposity suppression effect in mice due to black pepper and its main pungent component, piperine. Biosci. Biotechnol. Biochem. 2010, 74, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Melhorn, M.I.; Zandi, S.; Frimmel, S.; Tayyari, F.; Hisatomi, T.; Almulki, L.; Pronczuk, A.; Hayes, K.C.; Hafezi-Moghadam, A. An animal model of spontaneous metabolic syndrome: Nile grass rat. FASEB J. 2010, 24, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Demigne, C.; Bloch-Faure, M.; Picard, N.; Sabboh, H.; Besson, C.; Remesy, C.; Geoffroy, V.; Gaston, A.T.; Nicoletti, A.; Hagege, A.; et al. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur. J. Nutr. 2006, 45, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zhang, X.; Yu, H.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Metabolic syndrome exacerbates inflammation and bone loss in periodontitis. J. Dent. Res. 2015, 94, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Rahman, M.M.; Williams, P.J.; Fernandes, G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J. Nutr. Biochem. 2010, 21, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Du, Y.; Hang, S.; Chen, A.; Guo, F.; Xu, T. Adipocytes regulate the bone marrow microenvironment in a mouse model of obesity. Mol. Med. Rep. 2013, 8, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Maki, K. High-fat diet-induced obesity triggers alveolar bone loss and spontaneous periodontal disease in growing mice. BMC Obes. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.S.; Tintut, Y.; Parhami, F.; Kitchen, C.M.; Ivanov, Y.; Tetradis, S.; Effros, R.B. Bone density and hyperlipidemia: The T-lymphocyte connection. J. Bone Miner. Res. 2010, 25, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Pirih, F.; Lu, J.; Ye, F.; Bezouglaia, O.; Atti, E.; Ascenzi, M.G.; Tetradis, S.; Demer, L.; Aghaloo, T.; Tintut, Y. Adverse effects of hyperlipidemia on bone regeneration and strength. J. Bone Miner. Res. 2012, 27, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Pelton, K.; Krieder, J.; Joiner, D.; Freeman, M.R.; Goldstein, S.A.; Solomon, K.R. Hypercholesterolemia promotes an osteoporotic phenotype. Am. J. Pathol. 2012, 181, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.T.; Yau, S.K.; Mee, A.P.; Mawer, E.B.; Miller, C.A.; Garland, H.O.; Riccardi, D. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J. Am. Soc. Nephrol. 2001, 12, 779–790. [Google Scholar] [PubMed]
- Amir, G.; Rosenmann, E.; Sherman, Y.; Greenfeld, Z.; Ne’eman, Z.; Cohen, A.M. Osteoporosis in the Cohen diabetic rat: Correlation between histomorphometric changes in bone and microangiopathy. Lab. Investig. 2002, 82, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.F.; Brilhante, F.V.; Bezerra, J.P.; Silva, C.A.; Duarte, P.M. Trabecular bone area and bone healing in spontaneously hypertensive rats: A histometric study. Braz. Oral Res. 2010, 24, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nunez, N.P.; Carpenter, C.L.; Perkins, S.N.; Berrigan, D.; Jaque, S.V.; Ingles, S.A.; Bernstein, L.; Forman, M.R.; Barrett, J.C.; Hursting, S.D. Extreme obesity reduces bone mineral density: Complementary evidence from mice and women. Obesity 2007, 15, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Tintut, Y.; Beamer, W.G.; Gharavi, N.; Goodman, W.; Demer, L.L. Atherogenic high-fat diet reduces bone mineralization in mice. J. Bone Miner. Res. 2001, 16, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, C.; Devlin, M.J.; Rzeczkowska, P.A.; Herrmann, R.; Horsthemke, B.; Hauffa, B.P.; Grynpas, M.; Alm, C.; Bouxsein, M.L.; Palmert, M.R. Parental diabetes: The Akita mouse as a model of the effects of maternal and paternal hyperglycemia in wildtype offspring. PLoS ONE 2012, 7, e50210. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, X.; Wang, Q.; Wang, L. Hyperglycemia induces endoplasmic reticulum stress‑dependent CHOP expression in osteoblasts. Exp. Ther. Med. 2013, 5, 1289–1292. [Google Scholar] [PubMed]
- Metz, J.A.; Karanja, N.; Young, E.W.; Morris, C.D.; McCarron, D.A. Bone mineral density in spontaneous hypertension: Differential effects of dietary calcium and sodium. Am. J. Med. Sci. 1990, 300, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Hsu, J.F.; Jee, W.S.; Matthews, J.L. Evidence for reduced cancellous bone mass in the spontaneously hypertensive rat. Bone Miner. 1993, 20, 251–264. [Google Scholar] [CrossRef]
- Wright, G.L.; DeMoss, D. Evidence for dramatically increased bone turnover in spontaneously hypertensive rats. Metabolism 2000, 49, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Burghardt, A.J.; Yao, W.; Lane, N.E.; Majumdar, S.; Gullberg, G.T.; Seo, Y. Improved trabecular bone structure of 20-month-old male spontaneously hypertensive rats. Calcif. Tissue Int. 2014, 95, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yaturu, S.; Humphrey, S.; Landry, C.; Jain, S.K. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med. Sci. Monit. 2009, 15, Cr5–Cr9. [Google Scholar] [PubMed]
- Kim, H.Y.; Choe, J.W.; Kim, H.K.; Bae, S.J.; Kim, B.J.; Lee, S.H.; Koh, J.M.; Han, K.O.; Park, H.M.; Kim, G.S. Negative association between metabolic syndrome and bone mineral density in Koreans, especially in men. Calcif. Tissue Int. 2010, 86, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Choi, H.J. The relationship between low bone mass and metabolic syndrome in Korean women. Osteoporos. Int. 2010, 21, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, N.; Gao, Y.; Li, P.; Tian, M. Association between metabolic syndrome and osteoporotic fracture in middle-aged and elderly Chinese peoples. Cell Biochem. Biophys. 2014, 70, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, M.; Setoguchi, S.; Solomon, D.H. Bone mineral density in adults with the metabolic syndrome: Analysis in a population-based U.S. sample. J. Clin. Endocrinol. Metab. 2007, 92, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Metabolic syndrome and osteoporosis in relation to muscle mass. Calcif. Tissue Int. 2015, 97, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, S.L.; Barrett-Connor, E. Relation between body size and bone mineral density in elderly men and women. Am. J. Epidemiol. 1993, 138, 160–169. [Google Scholar] [PubMed]
- Pesonen, J.; Sirola, J.; Tuppurainen, M.; Jurvelin, J.; Alhava, E.; Honkanen, R.; Kroger, H. High bone mineral density among perimenopausal women. Osteoporos. Int. 2005, 16, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kanazawa, I.; Yamamoto, M.; Kurioka, S.; Yamauchi, M.; Yano, S.; Sugimoto, T. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone 2009, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Liu, Y.J.; Liu, P.Y.; Hamilton, J.; Recker, R.R.; Deng, H.W. Relationship of obesity with osteoporosis. J. Clin. Endocrinol. Metab. 2007, 92, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Fornari, R.; Rossi, F.; Santiemma, V.; Prossomariti, G.; Annoscia, C.; Aversa, A.; Brama, M.; Marini, M.; Donini, L. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int. J. Clin. Pract. 2010, 64, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Sellmeyer, D.E.; Ensrud, K.E.; Cauley, J.A.; Tabor, H.K.; Schreiner, P.J.; Jamal, S.A.; Black, D.M.; Cummings, S.R. Older women with diabetes have an increased risk of fracture: A prospective study. J. Clin. Endocrinol. Metab. 2001, 86, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K.L. Risk of fracture in women with type 2 diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Meilahn, E.; Zmuda, J.M.; Cauley, J.A. High blood pressure and bone-mineral loss in elderly white women: A prospective study. Study of Osteoporotic Fractures Research Group. Lancet 1999, 354, 971–975. [Google Scholar] [CrossRef]
- Gotoh, M.; Mizuno, K.; Ono, Y.; Takahashi, M. High blood pressure, bone-mineral loss and insulin resistance in women. Hypertens. Res. 2005, 28, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008, 32, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Grossmann, M.E. Obesity and breast cancer: The estrogen connection. Endocrinology 2009, 150, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Guzmán, G. Estrogen deficiency and the origin of obesity during menopause. BioMed Res. Int. 2014, 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Prestwood, K.M.; Kenny, A.M.; Kleppinger, A.; Kulldorff, M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: A randomized controlled trial. JAMA 2003, 290, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.M.; de Piano, A.; da Silva, P.L.; Carnier, J.; Sanches, P.L.; Corgosinho, F.C.; Masquio, D.C.; Lazaretti-Castro, M.; Oyama, L.M.; Nascimento, C.M.; et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine 2012, 42, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Honigmann, M.R.; Nath, A.K.; Murakami, C.; Garcia-Cardena, G.; Papapetropoulos, A.; Sessa, W.C.; Madge, L.A.; Schechner, J.S.; Schwabb, M.B.; Polverini, P.J.; et al. Biological action of leptin as an angiogenic factor. Science 1998, 281, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Robinson, C.E.; Wu, X.; Kelly, K.A.; Rodriguez, B.R.; Kliewer, S.A.; Lehmann, J.M.; Morris, D.C. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol. Pharmacol. 1996, 50, 1087–1094. [Google Scholar] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Czernik, B.; Moerman, E.J.; Grant, D.F.; Lehmann, J.M.; Manolagas, S.C.; Jilka, R.L. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 2002, 143, 2376–2384. [Google Scholar] [PubMed]
- Tian, L.; Yu, X. Lipid metabolism disorders and bone dysfunction—Interrelated and mutually regulated (review). Mol. Med. Rep. 2015, 12, 783–794. [Google Scholar] [PubMed]
- Das, S.; Crockett, J.C. Osteoporosis—A current view of pharmacological prevention and treatment. Drug Des. Dev. Ther. 2013, 7, 435–448. [Google Scholar]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Yao, W.; Cheng, Z.; Shahnazari, M.; Dai, W.; Johnson, M.L.; Lane, N.E. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J. Bone Miner. Res. 2010, 25, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Bennett, C.N.; Gerin, I.; Rapp, L.A.; Hankenson, K.D.; Macdougald, O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007, 282, 14515–14524. [Google Scholar] [CrossRef] [PubMed]
- Kayal, R.A.; Tsatsas, D.; Bauer, M.A.; Allen, B.; Al-Sebaei, M.O.; Kakar, S.; Leone, C.W.; Morgan, E.F.; Gerstenfeld, L.C.; Einhorn, T.A.; et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J. Bone Miner. Res. 2007, 22, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Botolin, S.; Faugere, M.C.; Malluche, H.; Orth, M.; Meyer, R.; McCabe, L.R. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology 2005, 146, 3622–3631. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Inoue, H. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Med. Hypotheses 2005, 65, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Petersen, K.F.; Shulman, G.I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006, 55, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Baylink, D.J. Impaired skeletal growth in mice with haploinsufficiency of IGF-I: Genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J. Endocrinol. 2005, 185, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xuan, S.; Bouxsein, M.L.; von Stechow, D.; Akeno, N.; Faugere, M.C.; Malluche, H.; Zhao, G.; Rosen, C.J.; Efstratiadis, A.; et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 2002, 277, 44005–44012. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S.; Mohamed, I.N.; Johari, M.H.; Ahmad, F.; Ramli, E.S.M.; Wan Ngah, W.Z. Insulin-like growth factor-1 is a mediator of age-related decline of bone health status in men. Aging Male 2014, 17, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Gauthier, B.; Trachtman, H. Hypercalciuria in children with renal glycosuria: Evidence of dual renal tubular reabsorptive defects. J. Pediatr. 1992, 121, 715–719. [Google Scholar] [CrossRef]
- Jackuliak, P.; Payer, J. Osteoporosis, fractures, and diabetes. Int. J. Endocrinol. Metab. 2014, 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Hypertension is a risk factor for fractures. Calcif. Tissue Int. 2009, 84, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium, and kidney stone risk. Nutr. Rev. 1995, 53, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, M.S.; Yatabe, J.; Takano, K.; Murakami, Y.; Sakuta, R.; Abe, S.; Sanada, H.; Kimura, J.; Watanabe, T. Effects of a high-sodium diet on renal tubule Ca2+ transporter and claudin expression in Wistar-Kyoto rats. BMC Nephrol. 2012, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Hata, M.; Taniguchi, J.; Tsuruoka, S.; Moriwaki, K.; Saitou, M.; Furuse, K.; Sasaki, H.; Fujimura, A.; Imai, M.; et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. USA 2010, 107, 8011–8016. [Google Scholar] [CrossRef] [PubMed]
- Alan, S.; Cheng, M.H.; Coalson, R.D. Calcium inhibits paracellular sodium conductance through claudin-2 by competitive binding. J. Biol. Chem. 2010, 285, 37060–37069. [Google Scholar]
- Ilic, K.; Obradovic, N.; Vujasinovic-Stupar, N. The relationship among hypertension, antihypertensive medications, and osteoporosis: A narrative review. Calcif. Tissue Int. 2013, 92, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Dennison, E.; Hindmarsh, P.; Fall, C.; Kellingray, S.; Barker, D.; Phillips, D.; Cooper, C. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J. Clin. Endocrinol. Metab. 1999, 84, 3058–3063. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Campino, C.; Carvajal, C.A.; Olivieri, O.; Guidi, G.; Faccini, G.; Vohringer, P.A.; Cerda, J.; Owen, G.; Kalergis, A.M.; et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin. Endocrinol. 2014, 80, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Wan, C.; Liu, Q.; Wang, Y.; Almeida, M.; O’Brien, C.A.; Thostenson, J.; Roberson, P.K.; Boskey, A.L.; Clemens, T.L. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 2010, 9, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Mantha, L.; Palacios, E.; Deshaies, Y. Modulation of triglyceride metabolism by glucocorticoids in diet-induced obesity. Am. J. Physiol. 1999, 277, R455–R464. [Google Scholar] [PubMed]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.M.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Wauters, M.A.; de Leeuw, I.H. The beneficial effects of modest weight loss on cardiovascular risk factors. Int. J. Obes. Relat. Metab. Disord. 1997, 21, S5–S9. [Google Scholar] [PubMed]
- Wing, R.R.; Koeske, R.; Epstein, L.H.; Nowalk, M.P.; Gooding, W.; Becker, D. Long-term effects of modest weight loss in type II diabetic patients. Arch. Intern. Med. 1987, 147, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Appel, L.J.; Espeland, M.A.; Applegate, W.B.; Ettinger, W.H., Jr.; Kostis, J.B.; Kumanyika, S.; Lacy, C.R.; Johnson, K.C.; Folmar, S.; et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: A randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998, 279, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2005, 353, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.C.; Gonzalez, D.; Mautalen, C.; Mazure, R.; Pedreira, S.; Vazquez, H.; Smecuol, E.; Siccardi, A.; Cataldi, M.; Niveloni, S.; et al. Long-term effect of gluten restriction on bone mineral density of patients with coeliac disease. Aliment. Pharmacol. Ther. 1997, 11, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, B.; Lee, H.; Choi, H.; Won, C. The Relationship between prevalence of osteoporosis and proportion of daily protein intake. Korean J. Fam. Med. 2013, 34, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Bonjour, J.P. Dietary protein and bone health. J. Bone Miner. Res. 2004, 19, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Haberle, L.; von Stengel, S. Effects of exercise on fracture reduction in older adults: A systematic review and meta-analysis. Osteoporos. Int. 2013, 24, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2011, 3, CD000333. [Google Scholar]
- Hopper, J.L.; Seeman, E. The bone density of female twins discordant for tobacco use. N. Engl. J. Med. 1994, 330, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Aspray, T.J.; Francis, R.M. Treatment of osteoporosis in women intolerant of oral bisphosphonates. Maturitas 2012, 71, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R.; Zhao, Y.-L.; Sun, X.-H.; Zhao, H.-X.; Tan, L.; Chen, D.-C.; Hai-Bin, X. Efficacy of intravenous zoledronic acid in the prevention and treatment of osteoporosis: A meta–analysis. Asian Pac. J. Trop. Med. 2012, 5, 743–748. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Marin, F.; Shane, E.; Dobnig, H.; Zanchetta, J.R.; Maricic, M.; Krohn, K.; See, K.; Warner, M.R. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: An analysis by gender and menopausal status. Osteoporos. Int. 2009, 20, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Dooley, C.; Della-Fera, M.A.; Hamrick, M.; Baile, C.A. Novel treatments for obesity and osteoporosis: Targeting apoptotic pathways in adipocytes. Curr. Med. Chem. 2005, 12, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Hart, D.J.; Spector, T.D. Oral statins and increased bone-mineral density in postmenopausal women. Lancet 2000, 355, 2218–2219. [Google Scholar] [CrossRef]
- Kondegowda, N.G.; Fenutria, R.; Pollack, I.R.; Orthofer, M.; Garcia-Ocana, A.; Penninger, J.M.; Vasavada, R.C. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-kappaB ligand pathway. Cell Metab. 2015, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Asaba, Y.; Ito, M.; Fumoto, T.; Watanabe, K.; Fukuhara, R.; Takeshita, S.; Nimura, Y.; Ishida, J.; Fukamizu, A.; Ikeda, K. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J. Bone Miner. Res. 2009, 24, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Nakagami, H.; Osako, M.K.; Nakagami, F.; Kunugiza, Y.; Tomita, T.; Yoshikawa, H.; Rakugi, H.; Ogihara, T.; Morishita, R. Prevention of osteoporosis by angiotensin-converting enzyme inhibitor in spontaneous hypertensive rats. Hypertens. Res. 2009, 32, 786–790. [Google Scholar] [CrossRef] [PubMed]

| Researcher (Year) | Types of Animal Model | Findings |
|---|---|---|
| Obesity | ||
| Nunez et al. 2007 [40] | Calorically dense diet-induced obese ovariectomized mice | ↑ body adiposity, ↑ leptin; ↓ BMD, ↓ BMC |
| Halade et al. 2010 [31] | HF diet-induced obese mice | ↑ body weight, ↑ total body fat mass, ↑ abdominal fat mass; ↓ BMD |
| Xu et al. 2013 [32] | HF diet-induced obese mice | ↓ tibia weight, ↓ BMD of tibia, fat cells accumulated in bone marrow of obese mice |
| Fujita & Maki 2015 [33] | HF diet-induced obese mice | ↑ body weight, ↑ total cholesterol, ↑ DL cholesterol, ↑ leptin, ↑triglyceride, ↑ Tb.Sp; ↓ BV/TV, ↓ Tb.N, ↓ Tb.Th |
| Dyslipidemia | ||
| Parhami et al. 2001 [41] | Atherogenic HF diet-induced hyperlipidemic mice | ↓ femoral mineral content, ↓ femoral mineral density, ↓ vertebral mineral content, ↓ osteocalcin |
| Graham et al. 2010 [34] | HF diet-induced hypercholesterolemic mice | ↑ total cholesterol, ↑ LDL, ↑ unesterified cholesterol; ↓ BMC value in femur and tibia, ↓ trabecular bone volume, thickness, and number |
| Pirih et al. 2012 [35] | HF diet-induced hyperlipidemic mice | ↓ cortical bone volume fraction (BV/TV), ↑ cortical porosity, ↓ bone strength and stiffness, ↓ PINP; ↑ PTH, ↑ TNF-α, ↑ CTX |
| Pelton et al. 2012 [36] | HFHC diet-induced hypercholesterolemic mice | ↑ triglyceride; ↓ BMD, ↓ failure load, ↓ energy to fracture |
| Hyperglycemia | ||
| Ward et al. 2001 [37] | STZ-induced diabetic rats | ↑ urinary calcium, ↓ bone formation marker |
| Amir et al. 2002 [38] | Cohen diabetic rat | ↓ BMD in distal femur and vertebra |
| Grasemann et al. 2012 [42] | Autosomal dominant diabetic mice (hypoinsulinemic hyperglycemia Akita mice) | ↓ body weight, impaired glucose tolerance, ↓ whole body BMD, ↓ trabecular bone mass |
| Liu et al. 2013 [43] | STZ-induced diabetic rats | ↓ BMD in femur, ↓ numbers of osteoblasts |
| Hypertension | ||
| Metz et al. 1990 [44] | SHR | ↓ BMD, ↓ bone magnesium |
| Wang et al. 1993 [45] | SHR (26-week-old) | ↓ body weight, ↓ BV/TV, ↓ Tb.Th, ↓ Tb.N, ↓ number of osteoblasts and osteoprogenitor cells; ↑ blood pressure, ↑ number of osteoclasts |
| Wright & DeMoss, 2000 [46] | SHR (24-week-old) | ↑ bone turnover in both male and female rats |
| Bastos et al. 2010 [39] | SHR | ↓ percentage of trabecular bone area, ↓ percentage of newly-formed bone area |
| Lee et al. 2014 [47] | SHR (20-month-old) | ↑ BV/TV, ↑ Tb.N; ↓ Tb.Sp |
| Researcher (Year) | Types of Study | Findings |
|---|---|---|
| Obesity | ||
| Edelstein & Barrett-Connor 1993 [54] | Rancho Bernardo Study (1492 ambulatory white adults, 55–84 years) | Body size, waist and hip ratio, BMI, and waist circumference were positively related with high BMD. |
| Jankowska et al. 2001 [12] | Polish men (272 men, 20–60 years) | Visceral adiposity (assessed by waist/hip ratio) contributed to reduced bone mass in men. |
| De Laet et al. 2005 [13] | 60,000 men and women from 12 cohorts Rotterdam, EVOS/EPOS, CaMos, Rochester, Sheffield, Dubbo, EPIDOS, OFELY, Kuopio, Hiroshima, and two cohorts from Gothenburg | Low BMI was associated with higher risk for all fractures. |
| Pesonen et al. 2005 [55] | Kuopio Osteoporosis Risk Factor and Prevention Study (1873 women, 48.0–59.6 years) | Premenopausal women had higher BMD, menopausal women had lower BMD. |
| Yamaguchi et al. 2009 [56] | 187 men (28–83 years) and 125 postmenopausal women (46–82 years) with type 2 diabetes | Visceral fat (men) and hyperinsulinemia (women) increased FN-BMD in diabetic, protecting against vertebral fracture. |
| Zhao et al. 2007 [57] | Chinese (878 pre-menopausal women, 1110 men; 19.6–45.1 years); Caucasian (2667 females, 1822 males; 19.1–90.1 years) | Increased fat mass did not have a beneficial effect on bone mass. |
| Greco et al. 2010 [58] | 398 obese patients (291 women, 107 men; age = 44.1 + 14.2 years) | Obese individuals had low lumbar BMD. |
| Dyslipidemia | ||
| Yamaguchi et al. 2002 [16] | 214 Japanese postmenopausal women (47–86 years) | High LDL and low HDL cholesterol levels caused low bone mass; high triglycerides levels caused low incidence of vertebral fractures in postmenopausal women. |
| Adami et al. 2004 [15] | 2 cohorts: 236 pre- or post-menopausal (35–81 years old); 265 men and 481 women (68–75 years) | The worse the lipid profile (lower HDL cholesterol and higher LDL cholesterol or triglycerides), the higher the bone mass. |
| Hyperglycemia | ||
| Barrett-Connor & Kritz-Silverstein 1996 [17] | Rancho Bernardo Heart and Chronic Disease Study (411 men and 559 women, 50–89 years) | Hyperinsulinemia only increased BMD in women, but not in men. |
| Schwartz et al. 2001 [59] | Osteoporotic Fractures Study (9654 women, ≥65 years) | Diabetic had increased risk of hip, proximal humerus, and foot fractures. |
| Hanley et al. 2003 [19] | Canadian Multicenter Osteoporosis Study (5566 women and 2187 men, ≥50 years) | Type II diabetes was associated with higher BMD in both men and women. |
| Bonds et al. 2006 [60] | Women’s Health Initiative Observational Cohort (93,676 postmenopausal women) | Women with type 2 diabetes were at increased risk for fractures. |
| Janghorbani et al. 2007 [61] | 836,941 participants from 16 eligible studies (two case-control studies and 14 cohort studies) | Type 1 and type 2 diabetes increased risk of hip fracture in men and women. |
| Yaturu et al. 2009 [48] | 3458 non-diabetic and 735 diabetic male veterans (50–76 years) | Diabetes lowered BMD resulted in increased incidence of hip fractures in men and higher osteoporosis. |
| Hypertension | ||
| Cappuccio et al. 1999 [62] | 3676 white women (66–91years) | Hypertension increased calcium losses which might contribute to hip fractures. |
| Hanley et al. 2003 [19] | Canadian Multicenter Osteoporosis Study (5566 women and 2187 men, ≥50 years) | Hypertension and type II diabetes were associated with higher BMD in both men and women. |
| Gotoh et al. 2005 [63] | 68 non-diabetic women with or without hypertension | Hypertension: ↓ BMD, ↑ calcium/sodium excretion ratio, ↑ PTH, ↑ 1,25(OH)2D. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. https://doi.org/10.3390/nu8060347
Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Ima-Nirwana S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients. 2016; 8(6):347. https://doi.org/10.3390/nu8060347
Chicago/Turabian StyleWong, Sok Kuan, Kok-Yong Chin, Farihah Hj Suhaimi, Fairus Ahmad, and Soelaiman Ima-Nirwana. 2016. "The Relationship between Metabolic Syndrome and Osteoporosis: A Review" Nutrients 8, no. 6: 347. https://doi.org/10.3390/nu8060347







