Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications
Abstract
:1. Introduction
2. Methods
| NCT Number | Study Type | Conditions | Study |
|---|---|---|---|
| NCT01331486 | Interventional | Prehypertension; Mild Hypertension | Nitric Oxide Mediated Vasodilatory Response to Hawthorn Standardized Extract |
| NCT00794456 | Interventional | Anxiety Disorder | Association of Passiflora Incarnata L; Crataegus Oxyacantha L and Salix Alba L. on Mild and Moderate Anxiety |
| NCT00006330 | Interventional | Heart Diseases | Pharmacokinetic and Pharmacodynamic Interaction Study of Digoxin and Hawthorn |
| NCT00343902 | Interventional | Chronic Heart Failure | Hawthorn Extract Randomized Blinded Chronic Heart Failure (HERB CHF) Trial |
| NCT01482819 | Interventional | Myopia | Evaluation of Daytime Corneal Swelling During Wear of Galyfilcon A Lenses |
| NCT00455026 | Interventional | Depth of Anesthesia | Effect of Remifentanil on Electroencephalographic BAR Index During Propofol Anesthesia |
| NCT00226837 | Interventional | Depth of Anesthesia | Quantifying Nitrous Oxide Effect on Depth of Anesthesia Using Theoretically Based Time Series Modelling |
| NCT01444287 | Interventional | Myopia | Daytime Corneal Swelling During Wear of Narafilcon B Lenses |
| NCT00762502 | Interventional | Astigmatism | Comparison of Senofilcon A Toric Lenses to Balafilcon A Toric Lenses Over |
| Extended Wear Period | |||
| NCT00027352 | Interventional | HIV Infections | A Comparison of Two Ways to Manage Anti-HIV Treatment (The SMART Study) |
3. Traditional Uses of C. Monogyna
4. Phytochemistry of C. Monogyna
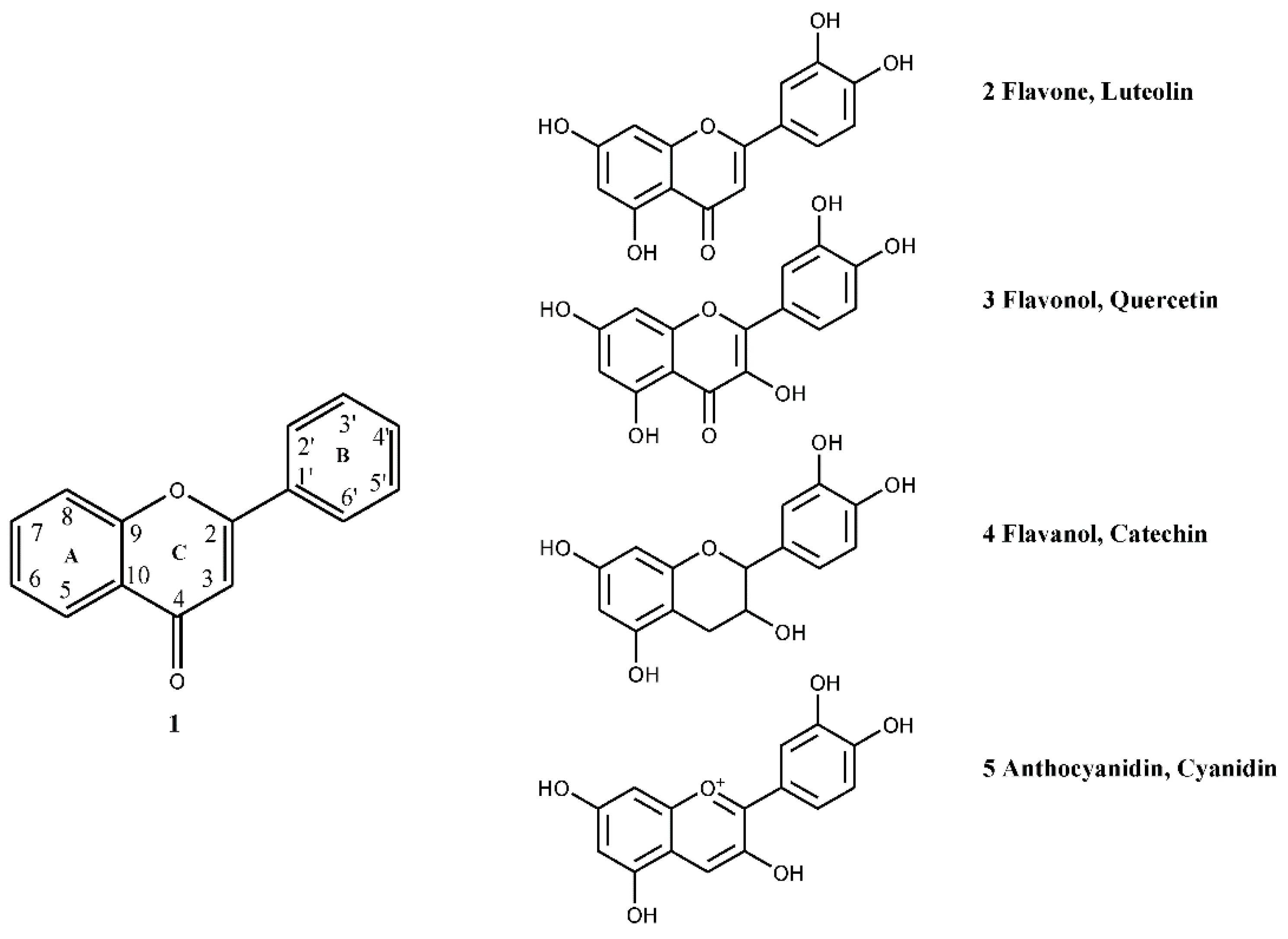
4.1. Flavonoids
4.1.1. Flavan-3-ols

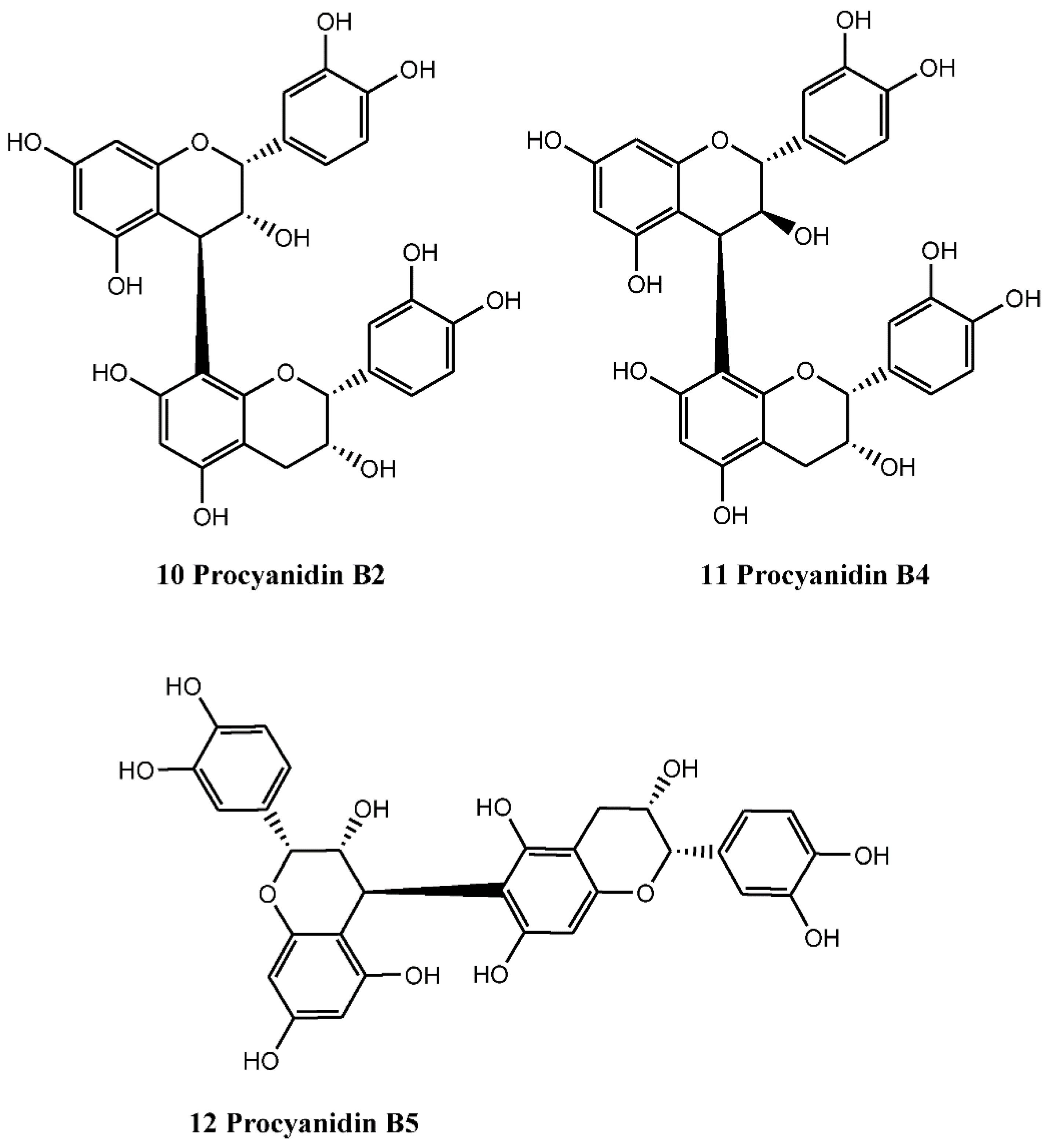
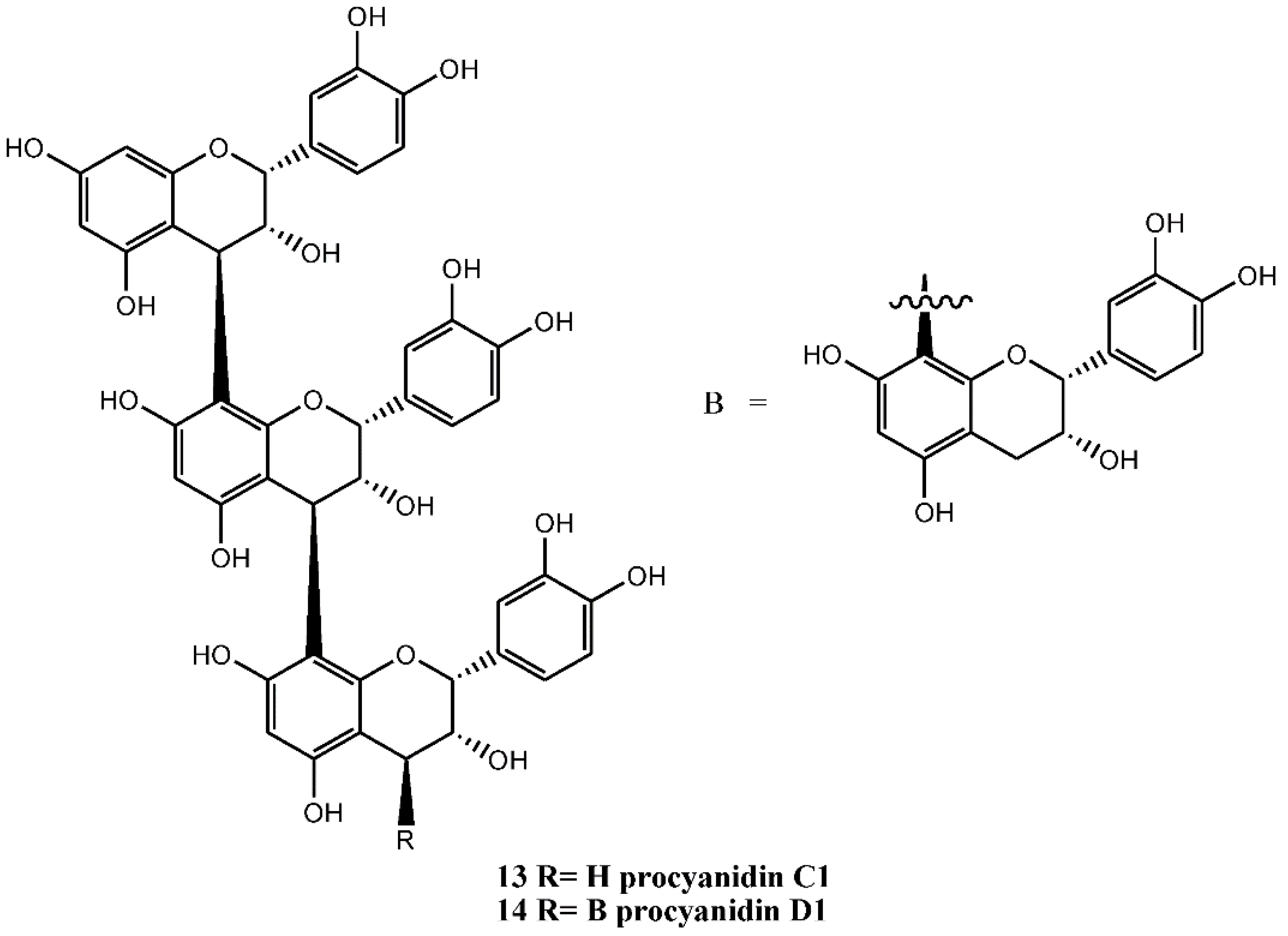
4.1.2. Procyanidins
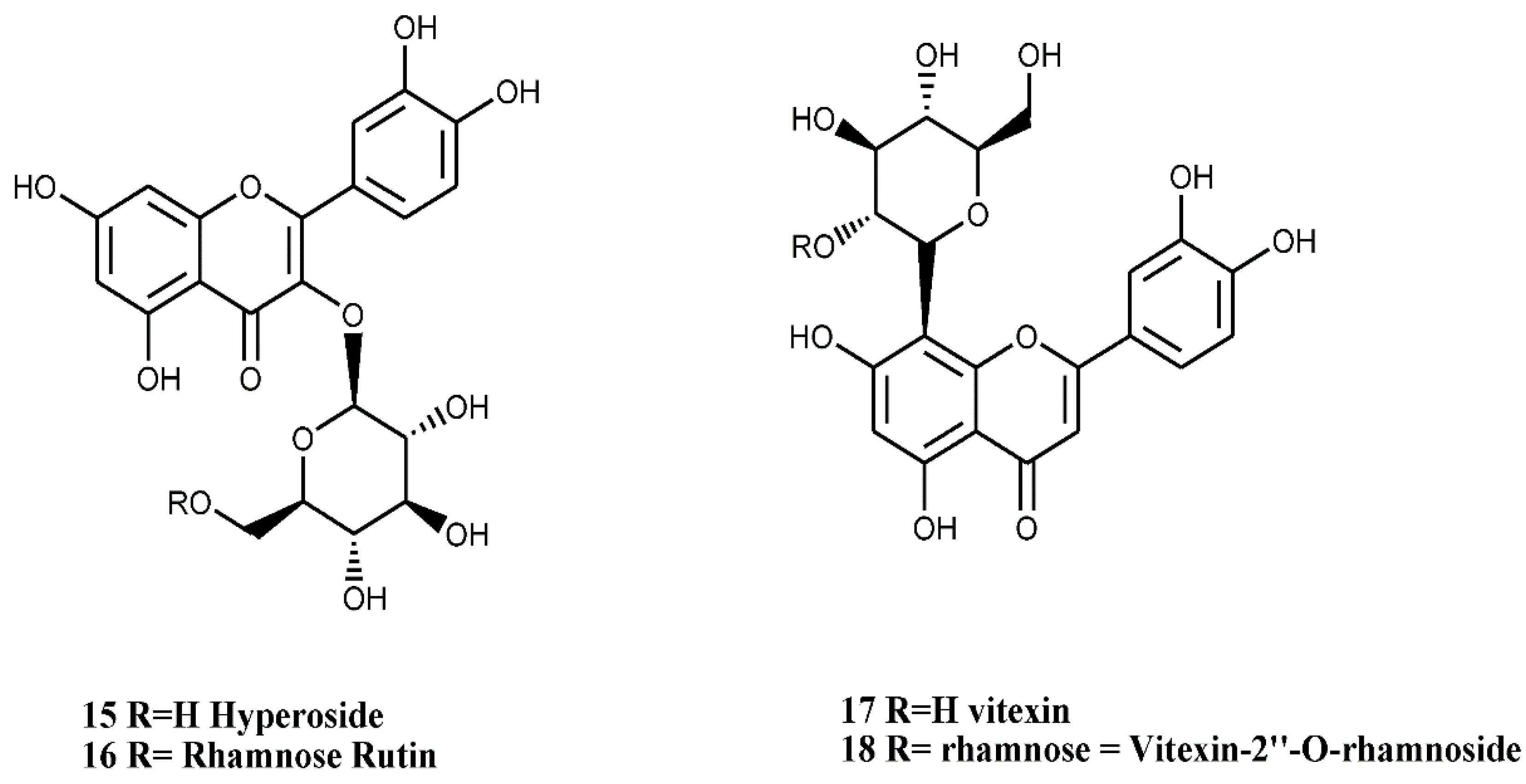
4.1.3. Flavones and Flavonols
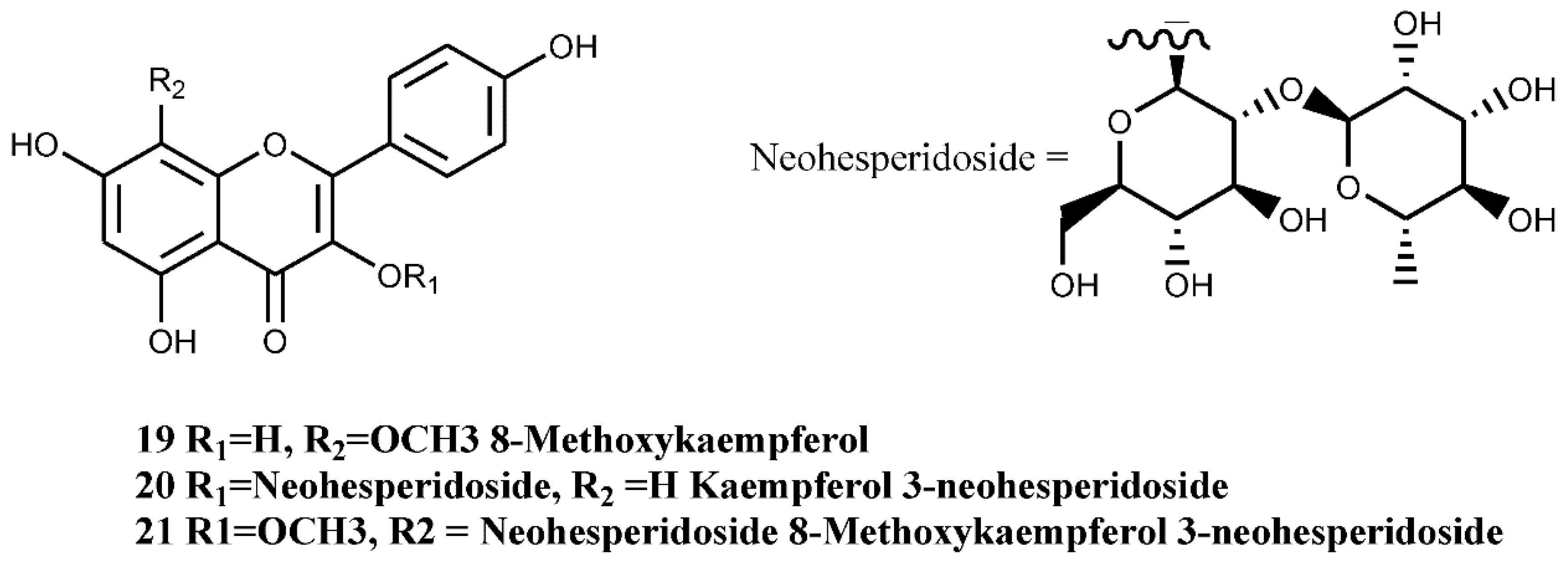

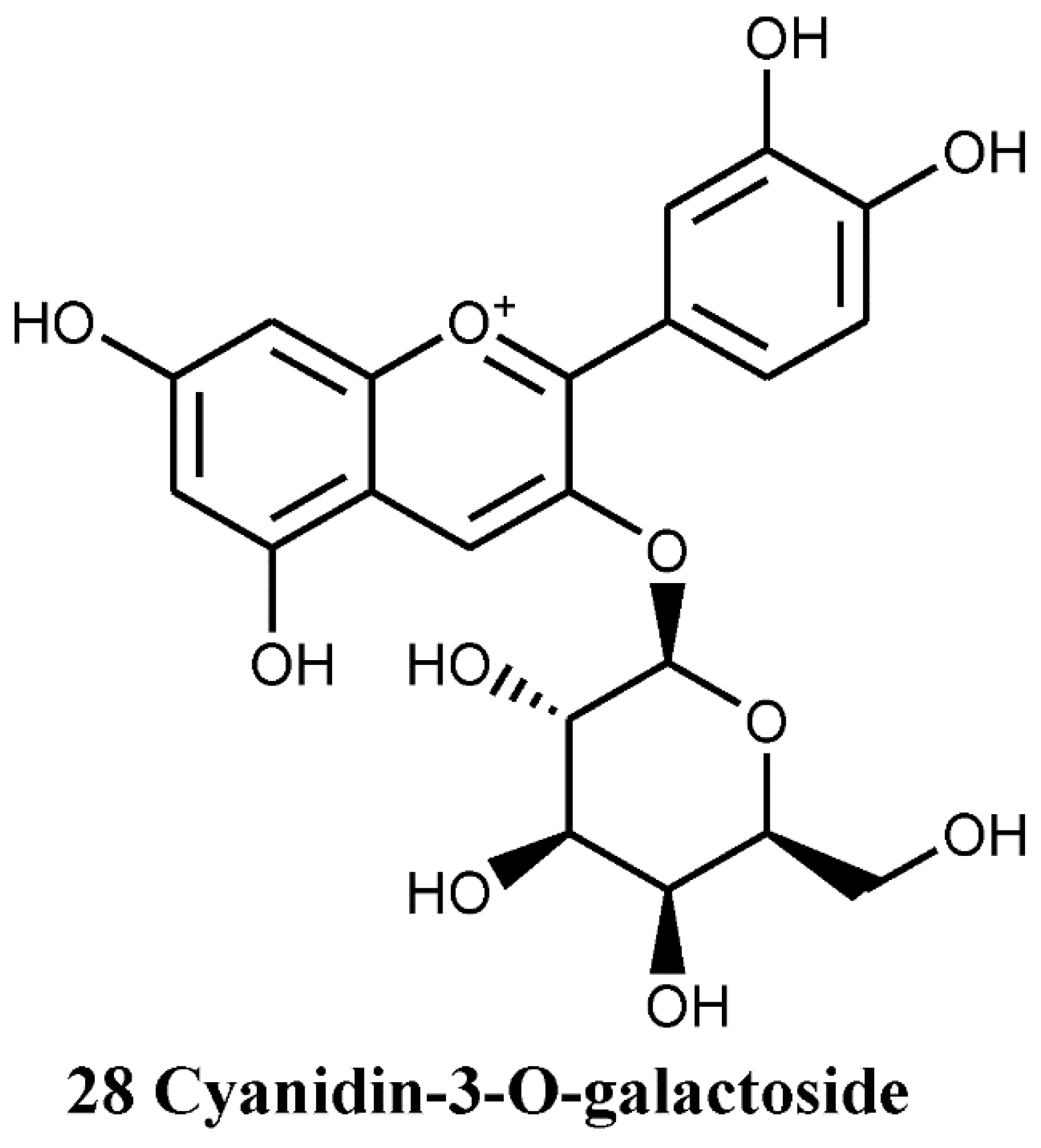
4.1.4. Anthocyanin and Anthocyanidins
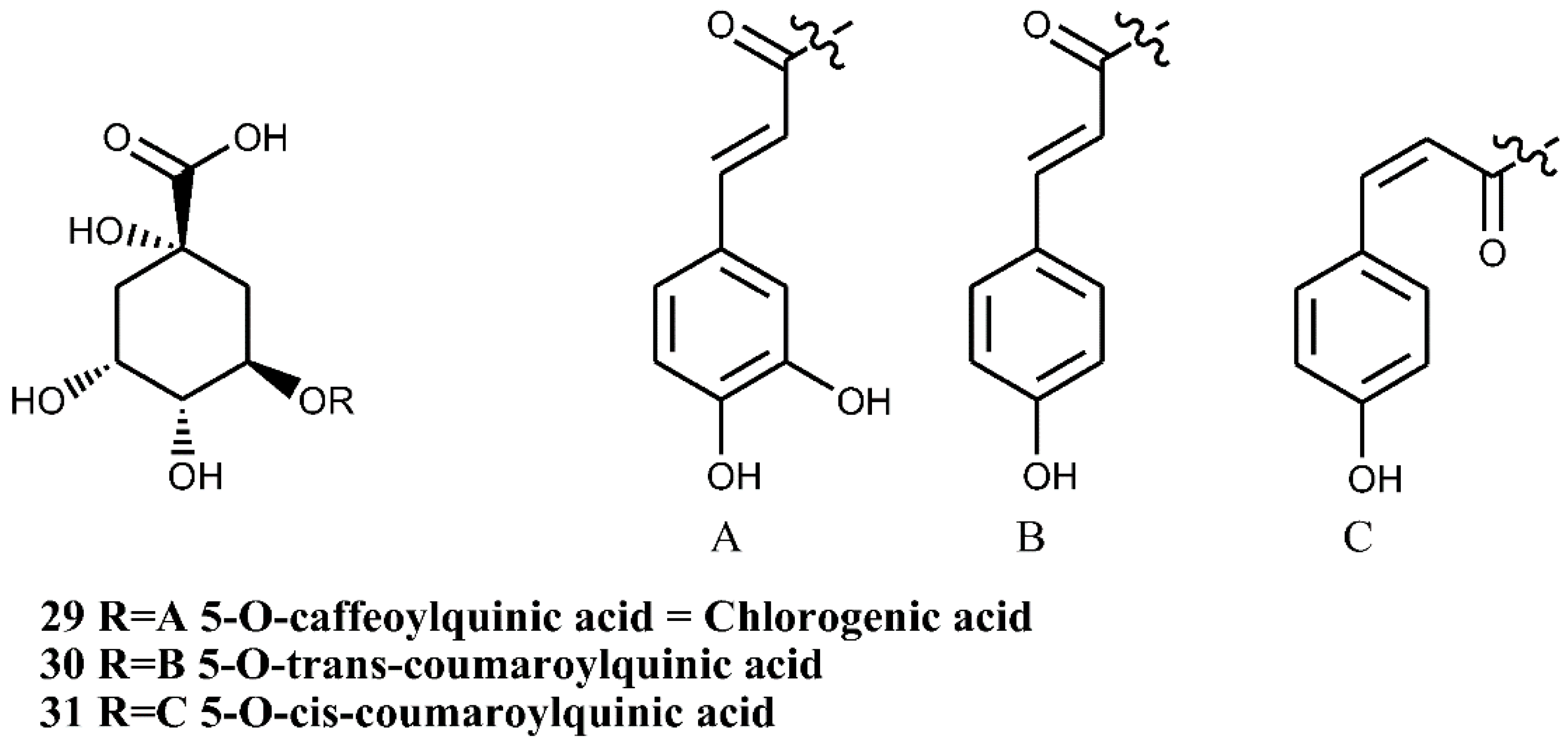
4.2. Chlorogenic Acids
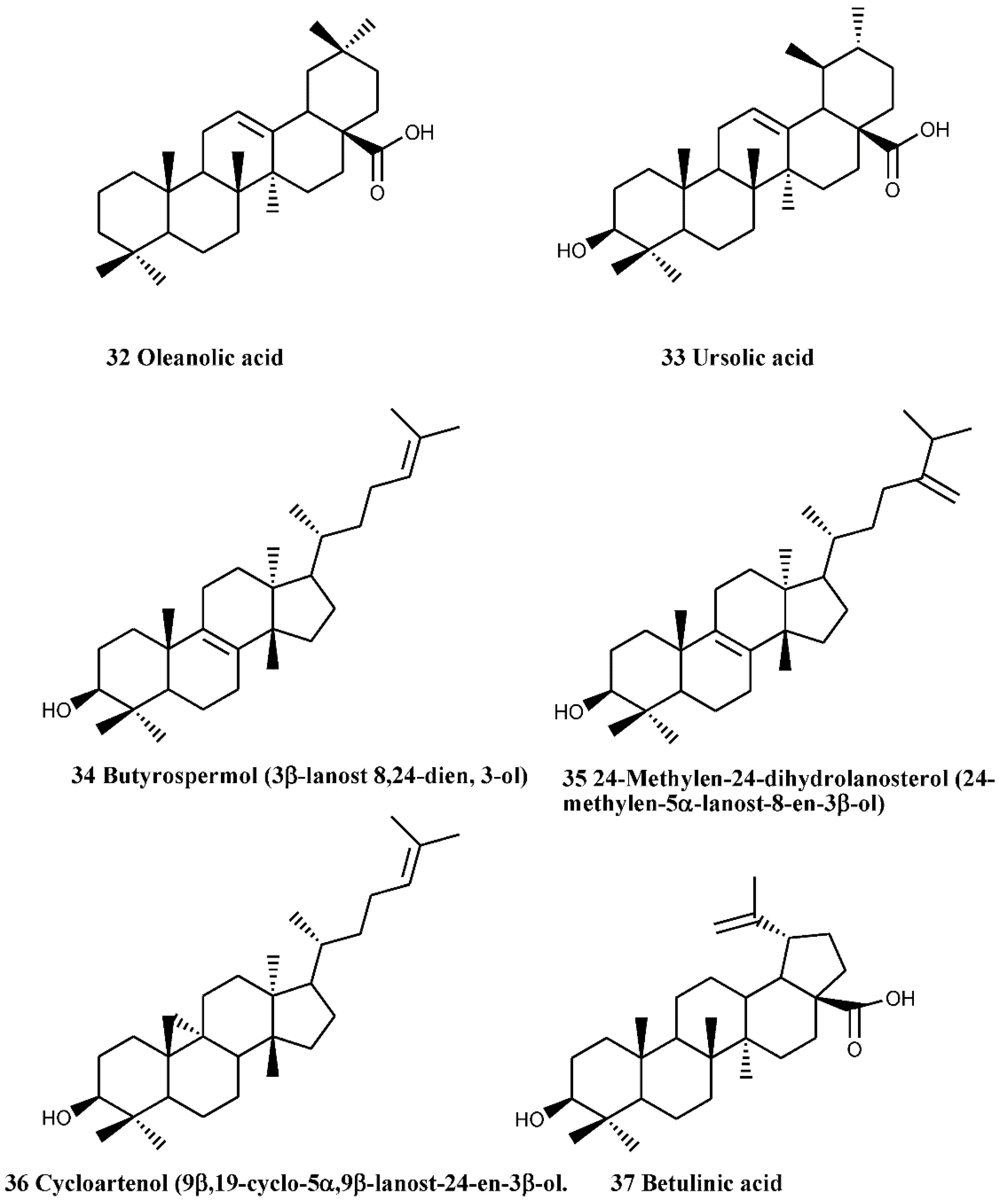
4.3. Triterpenes
5. Biological and Pharmacological Effects of C. monogyna Extracts
5.1. Cardiovascular Effects Registered in in Vitro and ex Vivo Experiments and in Animal Model Systems
5.2. Effect on the Nervous System
5.3. Other Beneficial Therapeutic Effects of C. Monogyna
5.4. Therapeutic Effects of Drug-Derived Toxicity
6. Adverse Effects/Toxicity of C. Monogyna
7. Drug Interactions
8. Clinical Impact of C. Monogyna
9. Conclusions
- (1)
- finding the best cultivation protocols and a way for increasing its production;
- (2)
- finding the bioactive constituents, which are the most responsible for its pharmacological effects and, thereafter, increasing its production through biotechnological protocols;
- (3)
- increasing the bioavailability of its bioactive constituents;
- (4)
- ascertaining the most effective dose for its clinical efficacy;
- (5)
- finding the exact molecular mechanisms responsible for its pharmacological effects.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nabavi, S.F.; Nabavi, S.M.; Moghaddam, A.H.; Naqinezhad, A.; Bigdellou, R.; Mohammadzadeh, S. Protective effects of Allium paradoxum against gentamicin-induced nephrotoxicity in mice. Food Funct. 2012, 3, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Nabavi, S.M.; Ebrahimzadeh, M.A.; Jafari, N.; Yazdanpanah, S. Biological Activities of Freshwater Algae, Spirogyra singularis Nordstedt. J. Aquat. Food Prod. Technol. 2013, 22, 58–65. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.; Setzer, W.; Nabavi, S.A.; Ebrahimzadeh, M.A. Antioxidant and antihemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits 2013, 68, 185–193. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Capelli, E.; Boschi, F.; Nabavi, S.F.; Bongiorno, A.I.; Habtemariam, S.; Nabavi, S.M.; Daglia, M. Modulation of human miR-17–3p expression by methyl 3-O-methyl gallate as explanation of its in vivo protective activities. Mol. Nutr. Food Res. 2014, 58, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Habtemariam, S.; Nabavi, S.F.; Sureda, A.; Daglia, M.; Moghaddam, A.H.; Amani, M.A. Protective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat’s kidney. Mol. Cell. Biochem. 2013, 372, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Eslami, S.; Moghaddam, A.H. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012, 132, 931–935. [Google Scholar] [CrossRef]
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Sallabanks, R. Fruit fate, frugivory, and fruit characteristics: A study of the hawthorn, Crataegus monogyna (Rosaceae). Oecologia 1992, 91, 296–304. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Hacıseferoğulları, H.; Marakoğlu, T.; Arslan, D. Hawthorn (Crataegus spp.) fruit: Some physical and chemical properties. J. Food Eng. 2005, 69, 409–413. [Google Scholar] [CrossRef]
- Bahorun, T.; Aumjaud, E.; Ramphul, H.; Rycha, M.; Luximon-Ramma, A.; Trotin, F.; Aruoma, O.I. Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Food/Nahrung 2003, 47, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J. Agric. Food Chem. 2003, 51, 3973–3976. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Dao, J.; Shao, Z.-H. Hawthorn: Potential roles in cardiovascular disease. Am. J. Chin. Med. 2005, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Long, S.R.; Proteau, P.J.; Filtz, T.M. Hawthorn (Crataegus monogyna Jacq.) extract exhibits atropine-sensitive activity in a cultured cardiomyocyte assay. J. Nat. Med. 2009, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine, PubMed database. Available online: http://www.ncbi.nlm.nih.gov/pubmed (accessed on 1 August 2015).
- U.S. National Institutes of Health, ClinicalTrials.gov. Available online: http://clinicaltrial.gov/ (accessed on 3 February 2015).
- Jalali, A.S.; Hasanzadeh, S.; Malekinejad, H. Crataegus monogyna aqueous extract ameliorates cyclophosphamide-induced toxicity in rat testis: Stereological evidences. Acta Med. Iran. 2012, 50, 1–8. [Google Scholar] [PubMed]
- Schmidt, U.; Kuhn, U.; Ploch, M.; Hübner, W.D. Efficacy of the hawthorn (Crataegus) preparation LI 132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine 1994, 1, 17–24. [Google Scholar] [CrossRef]
- Thompson, E.B.; Aynilian, G.H.; Gora, P.; Farnsworth, N.R. Preliminary study of potential antiarrhythmic effects of Crataegus monogyna. J. Pharm. Sci. 1974, 63, 1936–1937. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Bullitta, S.; Piluzza, G.; Viegi, L. Plant resources used for traditional ethnoveterinary phytotherapy in Sardinia (Italy). Genet. Resour. Crop Evol. 2007, 54, 1447–1464. [Google Scholar] [CrossRef]
- Fakir, H.; Korkmaz, M.; Güller, B. Medicinal plant diversity of western Mediterrenean region in Turkey. J. Appl. Biol. Sci. 2009, 3, 30–40. [Google Scholar]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Sokół-Łętowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Ðorđević, S.M.; Arsić, I.A.; Menković, N.A.R.; Stević, T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z.Y. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.; Santos-Buelga, C.; Ferreira, I.C. Crataegus monogyna buds and fruits phenolic extracts: Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Urbonavičiūtė, A.; Jakštas, V.; Kornyšova, O.; Janulis, V.; Maruška, A. Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. J. Chromatogr. A 2006, 1112, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-α in L-929 tumor cells. J. Nat. Prod. 1997, 60, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Natural inhibitors of tumour necrosis factor-alpha production, secretion and function. Planta Med. 2000, 66, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. A-glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011, 6, 201–203. [Google Scholar] [PubMed]
- Habtemariam, S.; Varghese, G. The antidiabetic therapeutic potential of dietary polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Trotin, F.; Vasseurt, J. Comparative polyphenolic productions in Crataegus monogyna callus cultures. Phytochemistry 1994, 37, 1273–1276. [Google Scholar] [CrossRef]
- Rohr, G.E.; Meier, B.; Sticher, O. Quantitative reversed-phase high-performance liquid chromatography of procyanidins in Crataegus leaves and flowers. J. Chromatogr. A 1999, 835, 59–65. [Google Scholar] [CrossRef]
- Nikolov, N.; DellaMonic, G.; Chopin, J. Di-C-glycosylflavones from Crataegus monogyna. Phytochemistry 1981, 20, 2780–2781. [Google Scholar] [CrossRef]
- Froehlicher, T.; Hennebelle, T.; Martin-Nizard, F.; Cleenewerck, P.; Hilbert, J.L.; Trotin, F.; Grec, S. Phenolic profiles and antioxidative effects of hawthorn cell suspensions, fresh fruits, and medicinal dried parts. Food Chem. 2009, 115, 897–903. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A simple GC-MS method for the screening of betulinic, corosolic, maslinic, oleanolic and ursolic acid contents in commercial botanicals used as food supplement ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Saenz, M.; Ahumada, M.; Cert, A. Isolation of three triterpenes and several aliphatic alcohols from Crataegus monogyna Jacq. J. Chromatogr. A 1997, 767, 340–342. [Google Scholar] [CrossRef]
- Rigelsky, J.M.; Sweet, B.V. Hawthorn: Pharmacology and therapeutic uses. Am. J. Health Syst. Pharm. 2002, 59, 417–422. [Google Scholar] [PubMed]
- Dahmer, S.; Scott, E. Health effects of hawthorn. Am. Fam. Physician 2010, 81, 465–468. [Google Scholar] [PubMed]
- Chu, C.Y.; Lee, M.J.; Liao, C.L.; Lin, W.L.; Yin, Y.F.; Tseng, T.H. Inhibitory effect of hot-water extract from dried fruit of Crataegus pinnatifida on low-density lipoprotein (LDL) oxidation in cell and cell-free systems. J. Agric. Food Chem. 2003, 51, 7583–7588. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.S.; Chen, C.T.; Chen, L.J.; Cheng, K.C.; Yeh, C.H.; Cheng, J.T. Decrease of Blood Lipids Induced by Shan-Zha (Fruit of Crataegus pinnatifida) is Mainly Related to an Increase of PPAR α in Liver of Mice Fed High-Fat Diet. Horm. Metab. Res. 2011, 43, 625–630. [Google Scholar] [PubMed]
- Kao, E.S.; Wang, C.J.; Lin, W.L.; Yin, Y.F.; Wang, C.P.; Tseng, T.H. Anti-inflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J. Agric. Food Chem. 2005, 53, 430–436. [Google Scholar]
- Li, C.; Son, H.J.; Huang, C.; Lee, S.K.; Lohakare, J.; Wang, M.H. Comparison of Crateagus pinnatifida Bunge var. typica Schneider and C. pinnatifida Bunge fruits for antioxidant, anti-alpha-glucoside, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 769–775. [Google Scholar] [CrossRef]
- Long, S.R.; Carey, R.A.; Crofoot, K.M.; Proteau, P.J.; Filtz, T.M. Effect of hawthorn (Crataegus oxyacantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine 2006, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, C.; Sáenz, T.; García, D.; De La Puerta, R.; Fernandez, A.; Martinez, E. The Effects of a Triterpene Fraction Isolated from Crataegus monogyna Jacq. on Different Acute Inflammation Models in Rats and Mice. Leucocyte Migration and Phospholipase A2 Inhibition. J. Pharm. Pharmacol. 1997, 49, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Schüssler, M.; Hölzl, J.; Fricke, U. Myocardial effects of flavonoids from Crataegus species. Arzneimittelforschung 1995, 45, 842–845. [Google Scholar] [PubMed]
- Attard, E.; Attard, H. The Potential Angiotensin-Converting Enzyme Inhibitory Activity of Oleanolic Acid in the Hydroethanolic Extract of Crataegus monogyna Jacq. Nat. Prod. Commun. 2006, 1, 381–386. [Google Scholar]
- Farrugia, D.L.; Shoemake, C.M.; Attard, E.; Azzopardi, L.M.; Mifsud, S.J. Investigative study on the angiotensin converting enzyme (ACE) inhibiting properties of the terpenoid extract of Crataegus monogyna using in silico models. J. Pharmacogn. Phytother. 2013, 5, 34–37. [Google Scholar]
- Arslan, R.; Bektas, N.; Bor, Z.; Sener, E. Evaluation of the antithrombotic effects of Crataegus monogyna and Crataegus davisii in the carrageenan-induced tail thrombosis model. Pharm. Biol. 2015, 53, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Zhang, Y.T.; Yin, J.J.; Zhao, B.L. Oral administration of Crataegus flavonoids protects against ischemia/reperfusion brain damage in gerbils. J. Neurochem. 2004, 90, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Elango, C.; Devaraj, S.N. Immunomodulatory effect of Hawthorn extract in an experimental stroke model. J. Neuroinflamm. 2010, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Elango, C.; Jayachandaran, K.S.; Niranjali Devaraj, S. Hawthorn extract reduces infarct volume and improves neurological score by reducing oxidative stress in rat brain following middle cerebral artery occlusion. Int. J. Dev. Neurosci. 2009, 27, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Can, O.D.; Ozkay, U.D.; Ozturk, N.; Ozturk, Y. Effects of hawthorn seed and pulp extracts on the central nervous system. Pharm. Biol. 2010, 48, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Congiu, F.; Tuberoso, C.I.; Amri, M.; Fattouch, S. Comparative analysis of Tunisian wild Crataegus azarolus (yellow azarole) and Crataegus monogyna (red azarole) leaf, fruit, and traditionally derived syrup: Phenolic profiles and antioxidant and antimicrobial activities of the aqueous-acetone extracts. J. Agric. Food Chem. 2013, 61, 9594–9601. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.L.; Grice, I.D.; Griffiths, L.R. Inhibition of platelet aggregation and 5-HT release by extracts of Australian plants used traditionally as headache treatments. Eur. J. Pharm. Sci. 2000, 9, 355–363. [Google Scholar] [CrossRef]
- João, C.M.; Barreira, J.C.M.; Rodrigues, S.; Carvalho, A.M.; Ferreira, I.C.F.R. Development of hydrosoluble gels with Crataegus monogyna extracts for topical application: Evaluation of antioxidant activity of the final formulations. Ind. Crop Prod. 2013, 42, 175–180. [Google Scholar]
- Zahra, A.; Gholamreza, N.; Farokhi, F.; Shalizar Jalali, A. Attenuation of cyclosporine-induced sperm impairment and embryotoxicity by Crataegus monogyna fruit aqueous extract. Cell J. 2013, 15, 198–205. [Google Scholar] [PubMed]
- Shalizar Jalali, A.; Hasanzadeh, S. Crataegus monogyna fruit aqueous extract as a protective agent against doxorubicin-induced reproductive toxicity in male rats. Avicenna J. Phytomed. 2013, 3, 159–170. [Google Scholar] [PubMed]
- Hosseinimehr, S.J.; Azadbakht, M.; Abadi, A.J. Protective effect of hawthorn extract against genotoxicity induced by cyclophosphamide in mouse bone marrow cells. Environ. Toxicol. Pharmacol. 2008, 25, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Azadbakht, M.; Tanha, M.; Mahmodzadeh, A.; Mohammadifar, S. Protective effect of hawthorn extract against genotoxicity induced by methyl methanesulfonate in human lymphocytes. Toxicol. Ind. Health 2011, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Mahmoudzadeh, A.; Azadbakht, M.; Akhlaghpoor, S. Radioprotective effects of Hawthorn against genotoxicity induced by gamma irradiation in human blood lymphocytes. Radiat. Environ. Biophys. 2009, 48, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, R.; Heywood, R. Toxicity of Crataegus (hawthorn) extract (WS 1442). Int. J. Toxicol. 1994, 13, 103–111. [Google Scholar] [CrossRef]
- Daniele, C.; Mazzanti, G.; Pittler, M.H.; Ernst, E. Adverse-event profile of Crataegus spp.: A systematic review. Drug Saf. 2006, 29, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Annon, J.; Cupp, M.J. Hawthorn in Toxicology and Clinical Pharmacology of Herbal Products; Humana Press: Totowa, New Jersey, USA, 2000; pp. 253–258. [Google Scholar]
- Holubarsch, C.J.; Colucci, W.S.; Meinertz, T.; Gaus, W.; Tendera, M. Survival and prognosis: Investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)-rationale, study design and study protocol. Eur. J. Heart Fail. 2000, 2, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Holubarsch, C.J.; Colucci, W.S.; Meinertz, T.; Gaus, W.; Tendera, M. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: The SPICE trial. Eur. J. Heart Fail. 2008, 10, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Zapfe, G. Clinical efficacy of crataegus extract WS 1442 in congestive heart failure NYHA class II. Phytomedicine 2001, 8, 262–266. [Google Scholar] [CrossRef]
- Degenring, F.H.; Suter, A.; Weber, M.; Saller, R. A randomised double blind placebo controlled clinical trial of a standardised extract of fresh Crataegus berries (Crataegisan®) in the treatment of patients with congestive heart failure NYHA II. Phytomedicine 2003, 10, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Vautaw, B.M.; Gillespie, B.; Aaronson, K.D. Hawthorn Extract Randomized Blinded Chronic Heart Failure (HERB CHF) trial. Eur. J. Heart Fail. 2009, 11, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.H.; Bauman, J.L. Hawthorn. J. Cardiovasc. Nurs. 2002, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.; Guo, R.; Ernst, E. Hawthorn extract for treating chronic heart failure. Cochrane Libr. 2008, 1, CD005312. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications. Nutrients 2015, 7, 7708-7728. https://doi.org/10.3390/nu7095361
Nabavi SF, Habtemariam S, Ahmed T, Sureda A, Daglia M, Sobarzo-Sánchez E, Nabavi SM. Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications. Nutrients. 2015; 7(9):7708-7728. https://doi.org/10.3390/nu7095361
Chicago/Turabian StyleNabavi, Seyed Fazel, Solomon Habtemariam, Touqeer Ahmed, Antoni Sureda, Maria Daglia, Eduardo Sobarzo-Sánchez, and Seyed Mohammad Nabavi. 2015. "Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications" Nutrients 7, no. 9: 7708-7728. https://doi.org/10.3390/nu7095361







