Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease
Abstract
:1. Introduction
2. Senescence
3. NADPH Oxidases in Aging
4. Mitochondrial Function in Aging
5. Polyphenols in Human Health
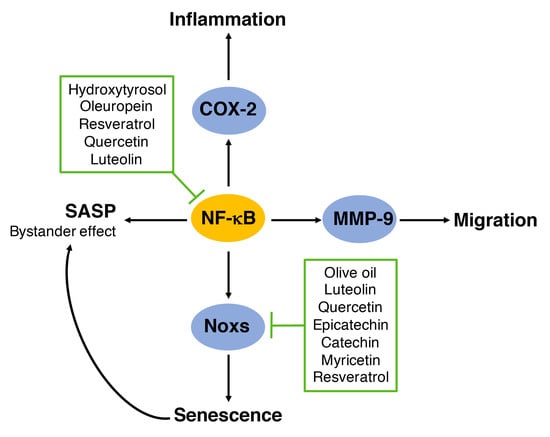
6. Regulation of NADPH Oxidases by Polyphenols
6.1. Structural Elements in Polyphenols Involved in NADPH Oxidase Function
6.2. Mediterranean Diet: Extra Virgin Olive Oil and Grapes
6.3. Polyphenols in Mitochondrial Function
6.4. Catechins in NADPH Oxidases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sieben, C.J.; Sturmlechner, I.; van de Sluis, B.; van Deursen, J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018, 28, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.L.; Jenkins, C.W.; Li, Y.; Nichols, M.A.; Wu, X.; O’Keefe, C.L.; Matera, A.G.; Xiong, Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994, 8, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Pereira-Smith, O.M. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006, 66, 8356–8360. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Prowse, K.R.; Tu, V.C.; Purdom, S.; Linskens, M.H. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts. Exp. Cell Res. 2001, 265, 294–303. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Sun, Y.; Coppe, J.P.; Lam, E.W. Cellular Senescence: The Sought or the Unwanted? Trends Mol. Med. 2018, 24, 871–885. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.E.; Humphry, M.; Bennett, M.R.; Clarke, M.C. Senescent Vascular Smooth Muscle Cells Drive Inflammation through an Interleukin-1alpha-Dependent Senescence-Associated Secretory Phenotype. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Boil. 2015, 17, 1049–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, A.; Patil, C.K.; Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Seo, K.W.; Yun, M.R.; Bae, S.S.; Lee, W.S.; Hong, K.W.; Kim, C.D. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-kappaB signaling pathways. Free Radic. Biol. Med. 2008, 45, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.; Huang, J.; Feresin, R.G.; Zhao, Y.; Griendling, K.K. Zinc regulates Nox1 expression through a NF-kappaB and mitochondrial ROS dependent mechanism to induce senescence of vascular smooth muscle cells. Free Radic. Biol. Med. 2017, 108, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.Y.; Campisi, J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; von Zglinicki, T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef]
- Calvert, P.A.; Liew, T.V.; Gorenne, I.; Clarke, M.; Costopoulos, C.; Obaid, D.R.; O’Sullivan, M.; Shapiro, L.M.; McNab, D.C.; Densem, C.G.; et al. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2157–2164. [Google Scholar] [CrossRef]
- Minamino, T.; Yoshida, T.; Tateno, K.; Miyauchi, H.; Zou, Y.; Toko, H.; Komuro, I. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation 2003, 108, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Cafueri, G.; Parodi, F.; Pistorio, A.; Bertolotto, M.; Ventura, F.; Gambini, C.; Bianco, P.; Dallegri, F.; Pistoia, V.; Pezzolo, A.; et al. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS ONE 2012, 7, e35312. [Google Scholar] [CrossRef] [PubMed]
- Balajee, A.S.; Geard, C.R. Replication protein A and gamma-H2AX foci assembly is triggered by cellular response to DNA double-strand breaks. Exp. Cell Res. 2004, 300, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Lassegue, B.; Clempus, R.E. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R277–R297. [Google Scholar] [CrossRef] [PubMed]
- Kigawa, Y.; Miyazaki, T.; Lei, X.F.; Kim-Kaneyama, J.R.; Miyazaki, A. Functional Heterogeneity of Nadph Oxidases in Atherosclerotic and Aneurysmal Diseases. J. Atheroscler. Thromb. 2017, 24, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, G. NADPH Oxidases and Mitochondria in Vascular Senescence. Int. J. Mol. Sci. 2018, 19, 1327. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.; Clempus, R.; Lassegue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Feresin, R.G.; Huang, J.; Klarich, D.S.; Zhao, Y.; Pourafshar, S.; Arjmandi, B.H.; Salazar, G. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food Funct. 2016, 7, 4175–4187. [Google Scholar] [CrossRef]
- Maheswaranathan, M.; Gole, H.K.; Fernandez, I.; Lassegue, B.; Griendling, K.K.; San Martin, A. Platelet-derived growth factor (PDGF) regulates Slingshot phosphatase activity via Nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J. Biol. Chem. 2011, 286, 35430–35437. [Google Scholar] [CrossRef]
- Li, Y.; Pagano, P.J. Microvascular NADPH oxidase in health and disease. Free Radic. Biol. Med. 2017, 109, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrowski, E.; Bender, B.; Huppert, J.; White, R.; Luhmann, H.J.; Kuhlmann, C.R. Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species. J. Vasc. Res. 2011, 48, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.A.; Thurman, G.; Gearheart, C.M.; Seewald, R.H.; Porter, C.C.; Ambruso, D.R. INF-gamma Enhances Nox2 Activity by Upregulating phox Proteins When Applied to Differentiating PLB-985 Cells but Does Not Induce Nox2 Activity by Itself. PLoS ONE 2015, 10, e0136766. [Google Scholar] [CrossRef] [PubMed]
- Helmcke, I.; Heumuller, S.; Tikkanen, R.; Schroder, K.; Brandes, R.P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox Signal. 2009, 11, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: Its regulation by oxidase organizers and activators. J. Biol. Chem. 2005, 280, 23328–23339. [Google Scholar] [CrossRef] [PubMed]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004, 18, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, J.P.; Spruce, C.A.; Fairfield, H.E.; Bergstrom, D.E. Generation of a conditional null allele of NADPH oxidase activator 1 (NOXA1). Genesis 2010, 48, 568–575. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Huang, X.; Man, Y.; Zhou, Y.; Wang, S.; Wang, J.; Li, J. NOX3-derived reactive oxygen species promote TNF-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 2010, 584, 995–1000. [Google Scholar] [CrossRef]
- Li, W.G.; Miller, F.J., Jr.; Zhang, H.J.; Spitz, D.R.; Oberley, L.W.; Weintraub, N.L. H2O2-induced O2 production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J. Biol. Chem. 2001, 276, 29251–29256. [Google Scholar] [CrossRef]
- Takac, I.; Schroder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef]
- Kussmaul, L.; Hirst, J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA 2006, 103, 7607–7612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziel, R.; Pircher, H.; Kratochwil, M.; Lener, B.; Hermann, M.; Dencher, N.A.; Jansen-Durr, P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem. J. 2013, 452, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010, 396, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Muller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Patrushev, N.; Forouzandeh, F.; Hilenski, L.; Alexander, R.W. PGC-1alpha Modulates Telomere Function and DNA Damage in Protecting against Aging-Related Chronic Diseases. Cell Rep. 2015, 12, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Salazar, G.; San Martin, A.; Ahmad, M.; Patrushev, N.; Hilenski, L.; Nazarewicz, R.R.; Ma, M.; Ushio-Fukai, M.; Alexander, R.W. PGC-1 alpha serine 570 phosphorylation and GCN5-mediated acetylation by angiotensin II drive catalase down-regulation and vascular hypertrophy. J. Biol. Chem. 2010, 285, 2474–2487. [Google Scholar] [CrossRef]
- Xiong, S.; Salazar, G.; Patrushev, N.; Ma, M.; Forouzandeh, F.; Hilenski, L.; Alexander, R.W. Peroxisome proliferator-activated receptor gamma coactivator-1alpha is a central negative regulator of vascular senescence. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 988–998. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Lai, H.T.; Threapleton, D.E.; Day, A.J.; Williamson, G.; Cade, J.E.; Burley, V.J. Fruit intake and cardiovascular disease mortality in the UK Women’s Cohort Study. Eur. J. Epidemiol. 2015, 30, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.H.; de Mejia, E.G.; Fan, J.; Lila, M.A.; Yousef, G.G. Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol. Nutr. Food Res. 2013, 57, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; Dos Santos, M.T.; Fank, B.; Dani, C.; Vanderlinde, R.; Henriques, J.A.; Coitinho, A.S.; Salvador, M. Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats. Nutr. Res. 2013, 33, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Peerson, J.M.; Freytag, T.L.; Breksa, A.P.; Bonnel, E.L.; Woodhouse, L.R.; Storms, D.H. Dietary grape powder increases IL-1beta and IL-6 production by lipopolysaccharide-activated monocytes and reduces plasma concentrations of large LDL and large LDL-cholesterol particles in obese humans. Br. J. Nutr. 2014, 112, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Kroon, P.A.; Saha, S.; de Biase, D.; D’Antuono, L.F.; Bordoni, A. Mixed pro- and anti-oxidative effects of pomegranate polyphenols in cultured cells. Int. J. Mol. Sci. 2014, 15, 19458–19471. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Borochov-Neori, H.; Judeinstein, S.; Aviram, M. Anti-atherogenic properties of date vs. pomegranate polyphenols: The benefits of the combination. Food Funct. 2015, 6, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Feresin, R.G.; Johnson, S.A.; Pourafshar, S.; Campbell, J.C.; Jaime, S.J.; Navaei, N.; Elam, M.L.; Akhavan, N.S.; Alvarez-Alvarado, S.; Tenenbaum, G.; et al. Impact of daily strawberry consumption on blood pressure and arterial stiffness in pre- and stage 1-hypertensive postmenopausal women: A randomized controlled trial. Food Funct. 2017, 8, 4139–4149. [Google Scholar] [CrossRef]
- D’Angelo, S.; La Porta, R.; Napolitano, M.; Galletti, P.; Quagliuolo, L.; Boccellino, M. Effect of Annurca apple polyphenols on human HaCaT keratinocytes proliferation. J. Med. Food 2012, 15, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Nemeth-Balogh, M.; Paulovicsova, B.; Bergstrom, A.; Wilcks, A.; Licht, T.R.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef]
- Vineetha, V.P.; Girija, S.; Soumya, R.S.; Raghu, K.G. Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food Funct. 2014, 5, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. [Google Scholar] [CrossRef]
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Suo, Y.; Chen, D.; Tong, L. Protection against vascular endothelial dysfunction by polyphenols in sea buckthorn berries in rats with hyperlipidemia. Biosci. Trends 2016, 10, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, M.; Palermo, V.; Bianchi, M.M.; Silvestri, R.; Falcone, C.; Tenore, G.; Novellino, E.; Mazzoni, C. Annurca apple (M. pumila Miller cv Annurca) extracts act against stress and ageing in S. cerevisiae yeast cells. BMC Complement. Altern. Med. 2017, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Bravo, K.; Duque, L.; Ferreres, F.; Moreno, D.A.; Osorio, E. Passiflora tarminiana fruits reduce UVB-induced photoaging in human skin fibroblasts. J. Photochem. Photobiol. B 2017, 168, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Stroher, D.J.; Escobar Piccoli Jda, C.; Gullich, A.A.; Pilar, B.C.; Coelho, R.P.; Bruno, J.B.; Faoro, D.; Manfredini, V. 14 Days of supplementation with blueberry extract shows anti-atherogenic properties and improves oxidative parameters in hypercholesterolemic rats model. Int. J. Food Sci. Nutr. 2015, 66, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, M.; Fuentes, E.; Olate, N.; Navarrete, S.; Carrasco, G.; Palomo, I. Strawberry extract presents antiplatelet activity by inhibition of inflammatory mediator of atherosclerosis (sP-selectin, sCD40L, RANTES, and IL-1beta) and thrombus formation. Platelets 2015, 26, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Moazen, S.; Amani, R.; Homayouni Rad, A.; Shahbazian, H.; Ahmadi, K.; Taha Jalali, M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2013, 63, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [Green Version]
- An, J.H.; Kim, D.L.; Lee, T.B.; Kim, K.J.; Kim, S.H.; Kim, N.H.; Kim, H.Y.; Choi, D.S.; Kim, S.G. Effect of Rubus Occidentalis Extract on Metabolic Parameters in Subjects with Prediabetes: A Proof-of-concept, Randomized, Double-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2016, 30, 1634–1640. [Google Scholar] [CrossRef]
- Suh, J.H.; Romain, C.; Gonzalez-Barrio, R.; Cristol, J.P.; Teissedre, P.L.; Crozier, A.; Rouanet, J.M. Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters. Food Funct. 2011, 2, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Pala, D.; Barbosa, P.O.; Silva, C.T.; de Souza, M.O.; Freitas, F.R.; Volp, A.C.P.; Maranhao, R.C.; Freitas, R.N. Acai (Euterpe oleracea Mart.) dietary intake affects plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: A prospective study in women. Clin. Nutr. 2018, 37, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; McDougall, G.J.M.; Al-Dujaili, E.A.S. Antioxidant Rich Potato Improves Arterial Stiffness in Healthy Adults. Plant Foods Hum. Nutr. 2018, 73, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Francesca, C.; Mario, M.; Alberto, F. Fruit and vegetables in hypertensive women with asymptomatic peripheral arterial disease. Clin. Nutr. ESPEN 2018, 27, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, C.; Castillo, E.; Mostaza, J.M.; de Dios, O.; Salinero-Fort, M.A.; Gonzalez-Alegre, T.; Garcia-Iglesias, F.; Estirado, E.; Laguna, F.; Sanchez, V.; et al. Relationship of the Adherence to a Mediterranean Diet and Its Main Components with CRP Levels in the Spanish Population. Nutrients 2018, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.; Jiang, X.; Tang, X.; Wu, Y.; Sun, K.; Xiang, X.; Tian, Y.; Wu, T.; Sun, Q.; Kraft, P.; et al. Joint Effects of PON1 Polymorphisms and Vegetable Intake on Ischemic Stroke: A Family-Based Case Control Study. Int. J. Mol. Sci. 2017, 18, 2652. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Yu, B.W.M.; Chan, R.S.M.; Leung, J. Influence of Dietary Patterns and Inflammatory Markers on Atherosclerosis Using Ankle Brachial Index as a Surrogate. J. Nutr. Health Aging 2018, 22, 619–626. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R.; et al. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide-mediated NADPH-oxidase 2 activation. Nutrition 2018, 55–56, 36–40. [Google Scholar] [CrossRef]
- Farras, M.; Fernandez-Castillejo, S.; Rubio, L.; Arranz, S.; Catalan, U.; Subirana, I.; Romero, M.P.; Castaner, O.; Pedret, A.; Blanchart, G.; et al. Phenol-enriched olive oils improve HDL antioxidant content in hypercholesterolemic subjects: A randomized, double-blind, cross-over, controlled trial. J. Nutr. Biochem. 2018, 51, 99–104. [Google Scholar] [CrossRef]
- Del Bo, C.; Deon, V.; Campolo, J.; Lanti, C.; Parolini, M.; Porrini, M.; Klimis-Zacas, D.; Riso, P. A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: Two randomized, controlled, crossover pilot studies. Food Funct. 2017, 8, 4108–4117. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Piao, Z.H.; Sun, S.; Liu, B.; Kim, G.R.; Seok, Y.M.; Lin, M.Q.; Ryu, Y.; Choi, S.Y.; Kee, H.J.; et al. Gallic Acid Reduces Blood Pressure and Attenuates Oxidative Stress and Cardiac Hypertrophy in Spontaneously Hypertensive Rats. Sci. Rep. 2017, 7, 15607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem. Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martinez-Gonzalez, M.A.; Estruch, R.; Salas-Salvado, J.; Fito, M.; Martinez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-alpha-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-kappaB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IKappaBalpha/NF-kappaB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef]
- Lamy, S.; Ouanouki, A.; Beliveau, R.; Desrosiers, R.R. Olive oil compounds inhibit vascular endothelial growth factor receptor-2 phosphorylation. Exp. Cell Res. 2014, 322, 89–98. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCalpha and PKCbeta1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Rosello-Catafau, J.; Pinto, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Boil. 2014, 2, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Adachi, Y.; Mitsushita, J.; Kuwabara, M.; Nagasawa, A.; Harada, S.; Furuta, S.; Zhang, Y.; Seheli, K.; Miyazaki, H.; et al. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J. Biol. Chem. 2010, 285, 4481–4488. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.S.; Eligini, S.; Brambilla, M.; Tremoli, E.; Colli, S. Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: Critical role of NADPH oxidase. Cardiovasc. Res. 2003, 60, 187–197. [Google Scholar] [CrossRef]
- Davalos, A.; de la Pena, G.; Sanchez-Martin, C.C.; Teresa Guerra, M.; Bartolome, B.; Lasuncion, M.A. Effects of red grape juice polyphenols in NADPH oxidase subunit expression in human neutrophils and mononuclear blood cells. Br. J. Nutr. 2009, 102, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60 (Suppl. 4), 111–116. [Google Scholar]
- Schilder, Y.D.; Heiss, E.H.; Schachner, D.; Ziegler, J.; Reznicek, G.; Sorescu, D.; Dirsch, V.M. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic. Biol. Med. 2009, 46, 1598–1606. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Qu, W.; Peng, X.; Xin, P.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem. 2012, 23, 1410–1416. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Q.; Song, L. Resveratrol enhances matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage. Biosci. Rep. 2018, 38, BSR20180544. [Google Scholar] [CrossRef] [PubMed]
- Alayev, A.; Doubleday, P.F.; Berger, S.M.; Ballif, B.A.; Holz, M.K. Phosphoproteomics reveals resveratrol-dependent inhibition of Akt/mTORC1/S6K1 signaling. J. Proteome Res. 2014, 13, 5734–5742. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, C.E.; Kumerz, M.; Gesslbauer, J.; Schachner, D.; Joa, H.; Erker, T.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M. Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc. Res. 2011, 90, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Martin, K.A.; Rzucidlo, E.M. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS ONE 2014, 9, e85495. [Google Scholar] [CrossRef] [PubMed]
- Haider, U.G.; Sorescu, D.; Griendling, K.K.; Vollmar, A.M.; Dirsch, V.M. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol. Pharmacol. 2002, 62, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Miyazaki, R.; Kamiharaguchi, A.; Hashimoto, T.; Matsuura, H.; Kitamoto, S.; Tokunou, T.; Sunagawa, K. Resveratrol attenuates angiotensin II-induced senescence of vascular smooth muscle cells. Regul. Pept. 2012, 177, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Fischer, S.M. Black tea polyphenols inhibit IGF-I-induced signaling through Akt in normal prostate epithelial cells and Du145 prostate carcinoma cells. Carcinogenesis 2002, 23, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duluc, L.; Jacques, C.; Soleti, R.; Andriantsitohaina, R.; Simard, G. Delphinidin inhibits VEGF induced-mitochondrial biogenesis and Akt activation in endothelial cells. Int. J. Biochem. Cell Biol. 2014, 53, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Zhang, Y. Targeting Autophagy in Aging and Aging-Related Cardiovascular Diseases. Trends Pharmacol. Sci. 2018, 39, 1064–1076. [Google Scholar] [CrossRef]
- Teixeira, J.; Deus, C.M.; Borges, F.; Oliveira, P.J. Mitochondria: Targeting mitochondrial reactive oxygen species with mitochondriotropic polyphenolic-based antioxidants. Int. J. Biochem. Cell Biol. 2018, 97, 98–103. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Fusi, J.; Bianchi, S.; Daniele, S.; Pellegrini, S.; Martini, C.; Galetta, F.; Giovannini, L.; Franzoni, F. An in vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed. Pharmacother. 2018, 101, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Oteiza, P.I. (-)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells. Arch. Biochem. Biophys. 2018, 646, 55–63. [Google Scholar] [CrossRef]
- Liu, L.; Nagai, I.; Gao, Y.; Matsushima, Y.; Kawai, Y.; Sayama, K. Effects of catechins and caffeine on the development of atherosclerosis in mice. Biosci. Biotechnol. Biochem. 2017, 81, 1948–1955. [Google Scholar] [CrossRef]
- Mika, M.; Kostogrys, R.B.; Franczyk-Zarow, M.; Wikiera, A.; Maslak, E. Anti-atherosclerotic activity of catechins depends on their stereoisomerism. Atherosclerosis 2015, 240, 125–130. [Google Scholar] [CrossRef]
- Mangels, D.R.; Mohler, E.R., 3rd. Catechins as Potential Mediators of Cardiovascular Health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef]
- Chen, X.Q.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.B.; Zhang, Y.T.; Du, Y.; Jiang, Y.W. Preventive Effects of Catechins on Cardiovascular Disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Cremonini, E.; Kang, H.; Kang, J.; Haj, F.G.; Oteiza, P.I. Anti-inflammatory actions of (-)-epicatechin in the adipose tissue of obese mice. Int. J. Biochem. Cell Biol. 2016, 81, 383–392. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Boil. 2018, 18, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (−)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Boil. 2018, 14, 588–599. [Google Scholar] [CrossRef]
- Kabirifar, R.; Ghoreshi, Z.A.; Safari, F.; Karimollah, A.; Moradi, A.; Eskandari-Nasab, E. Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 88–95. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef]
- Bouras, T.; Fu, M.; Sauve, A.A.; Wang, F.; Quong, A.A.; Perkins, N.D.; Hay, R.T.; Gu, W.; Pestell, R.G. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 2005, 280, 10264–10276. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.W.; Ferguson, B.S. Food Bioactive HDAC Inhibitors in the Epigenetic Regulation of Heart Failure. Nutrients 2018, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Gurau, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafe, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef] [PubMed]




| Author, Year | Treatment | Model | Result | Conclusion |
|---|---|---|---|---|
| Lai et al. 2015 [50] | Report of total fruit intake using food frequency questionnaire (FFQ) over an average of 16.7 years | 30,458 women (35–69 years old) | Total fruit intake was associated with reduced risk for cardiovascular disease (CVD) mortality | Fruit intake shows health improvements in women for CVD and stroke |
| Zunino et al. 2014 [53] | 46 g of grape powder or placebo twice daily for 3 weeks. | 24 obese (8 men, 16 women; 20–60 years old) | Significant decrease in low-density lipoprotein (LDL) cholesterol and increase in immune cell function | Grapes may improve cholesterol levels and immune response in obese individuals |
| Ravn-Haren et al. 2013 [58] | 550 g/day WA, 22 g/day AP or 500 mL/day of either clear or cloudy AJ | 5 × 4 weeks cross-over study in 23 healthy volunteers | Significant decrease in total cholesterol (TC) and LDL cholesterol in WA and AP but not AJ | The fiber content in WA and AP mediates the effect of apples in cholesterol levels |
| Huebbe et al. 2012 [60] | Blackcurrant juice compared to red grape, raspberry, and cherry juice In vitro 0.2 mg/mL for 4 h In vivo 250 g fruit beverage with high energy meal | In vitro murine macrophage RAW264.7 In vivo human subjects with atherosclerosis prone phenotype 11 men (mean age 37.4) | In vitro, decreased TNFα, IL-1β and iNOS mRNA levels In vivo, increased radical-scavenging capacity and decreased triglycerides | Blackcurrant juice may reduce atherosclerosis by decreasing triglycerides and inflammation related to NF-κB |
| Karlsen et al. 2010 [61] | In vivo, 4 weeks treatment with 330 mL/day bilberry juice (BBJ) In vitro BBJ purified polyphenols | In vivo, 62 human subjects with at least one CVD risk factor. In vitro, human monocytic cell line stimulated with LPS | In vivo reduction in CRP, IL-6, IL-15 In vitro epicatechin, and resveratrol strongly inhibited NF-κB activation. | BBJ decreased NF-κB activity in individuals at high risk of CVD. In vitro polyphenols inhibited NF-κB |
| Yang et al. 2016 [62] | Polyphenol extract of sea buckthorn berry administered orally. 7, 14, or 28 mg/kg for 5 weeks | 4-week old male Sprague Dawley rats with hyperlipidemia from high-fat diet (HFD) | Reduced serum lipids, TNFα, IL-6, eNOS, and intercellular adhesion molecule-1 (ICAM) and increased superoxide dismutases (SOD) and glutathione peroxidases (GPx) activity | Sea buckthorn berry polyphenols reduce inflammation and increase antioxidant capacity |
| Rosenblat et al. 2015 [55] | Consumption of 0.5 μmol gallic acid/day from pomegranate juice, Hallawi or date seed extract, or a combination for 3 weeks | 6-week old male ApoE−/− mice | Combination treatment decreased total triglycerides, and oxidative stress, and lipid peroxidation in macrophages in aortas | The most effective reduction of lipids and oxidative stress was found by using a combination of polyphenols from various sources |
| Stirpe et al. 2017 [63] | Annurca apple extract (1 and 10 mg/mL) for 3 days | S. cerevisiae strain MCY4/Kllsm4Δ1 | Significant decrease in reactive oxygen species (ROS) levels and DNA damage and increased viability | Apple extract increases lifespan by reducing oxidative stress |
| Bravo et al. 2016 [64] | Cells treated with <10 μg/mL of Passiflora tarminiana extract (620 mg po-lyphenol/g for 24 h | Human diploid fibroblasts treated with UV radiation | Reduced collagen degradation, matrix metalloproteinase (MMP-1) expression and ROS levels | P. tarminiana reduces UVB photoaging in human skin fibroblasts |
| Stroher et al. 2015 [65] | Supplementation of hyperlipidemic diet with 25 or 50 mg/kg blueberry extract for 14 days | 36 one-month old male Wistar rats | Reduced aortic lesions, oxidative damage and increased antioxidant capacity | Blueberry extract improved lipid profile, antioxidant defense, and reduced oxidative stress |
| Wu et al. 2010 [66] | AIN-93G high-fat diet supplemented with 1% freeze dried blueberry for 20 weeks | 4-month old female ApoE−/− mice | Decreased lipid peroxidation and atherosclerotic lesions and increased antioxidant capacity | Blueberry reduced oxidative stress and protected against atherosclerosis |
| Alarcon et al. 2015 [67] | In vitro platelets treated with strawberry extract. In vivo strawberry extracts injected intraperitoneally in mice | In vitro platelets from 6 healthy volunteers In vivo C57BL/6 mice 12-16-week old with photochemical induced thrombosis | Reduced platelet aggregation in vitro and thrombus formation in vivo | Strawberry extract inhibits thrombus formation and targets platelet activation responses |
| Moazen et al. 2013 [68] | Daily consumption of 2 cups freeze-dried strawberries (FDS) containing 154 mg anthocyanins for 6 weeks | 23 females with T2D average age 51.5 years | Reduced C-reactive protein (CRP), lipid peroxidation, and HbA1c and increased total antioxidant status | Strawberry improved glycemic control, and reduced inflammation and oxidative stress in T2D patients |
| Basu et al. 2010 [69] | Consumption of 4 cups of FDS beverage daily for 8 weeks | 27 subjects (2 male 25 female, mean age 47) with metabolic syndrome | Decreased total and LDL-cholesterol, and vascular cell adhesion molecule 1 (VCAM-1) levels | Strawberry improved CVD risk factors including dyslipidemia and VCAM-1 level |
| An et al. 2016 [70] | Consumption of Rubus occidentalis extract at low (900 mg/day) or high dose (1800 mg/day) for 12 weeks | 44 prediabetic patients (31 female, 13 male, mean age 59 years) | High dose reduced glucose levels, while MCP-1 and oxidized LDL were reduced in a dose dependent manner | Rubus occidentalis improved glycemic control and vascular inflammation in prediabetic patients |
| Suh et al. 2011 [71] | Consumption of atherogenic diet with a daily dose of cranberry juice varieties for 12 weeks | 10 weanling male Syrian golden hamsters | Reduced superoxide, plasma triglycerides, and increased GPx. Cardinal juice, decreased LDL and increased high-density lipoprotein (HDL) level | Consuming various cranberry juices can reduce atherosclerosis by increasing antioxidant capacity and improving lipid profile |
| Pala et al. 2018 [72] | Daily consumption of 200 g of acai pulp for 4 weeks | 40 healthy women average age 24 years | Acai increased total antioxidants and cholesterol in HDL. Decreased ROS and oxidized LDL levels | Acai fruit reduced oxidative stress and improved cholesterol transport that could reduce atherosclerosis |
| Author, Year | Treatment | Model | Result | Conclusion |
|---|---|---|---|---|
| Tsang et al. 2018 [73] | Consumption of cooked purple potato (200 g/day containing 288 mg anthocyanins) for 14 days | 14 healthy, non-smoking male and female adults age 22–55 years | Decreased pulse wave velocity compared to white potato | Purple majesty potato may reduce arterial stiffness, an independent indicator of CVD |
| Mattioli et al. 2018 [74] | Self-reported fruit and vegetable intake through FFQ Ankle-brachial index (ABI) was used to assess preclinical atherosclerosis | 237 women with hypertension age 45–54 years | Fruit and vegetable intake was associated with reduced ABI, while vegetable intake was associated with reduced risk of periphery artery disease | Fruit and vegetable consumption is associated with a lower risk of atherosclerosis in pre-menopausal women |
| Lahoz et al. 2010 [75] | Adherence to a Mediterranean diet based on the Mediterranean diet adherence screener | 1411 subjects (43% male, mean age 61 years) | Inverse relationship between Mediterranean diet and CRP levels | Mediterranean diet, rich in fruits and vegetables, decreases atherosclerotic risk factors |
| Juan et al. 2017 [76] | Fruit and vegetable intake reported by FFQ in in-person interviews | Case study of 2158 Chinese subjects (918 families) testing association of PON1 rs662 polymorphism with risk of stroke | Individuals with PON1 rs662 gene reduced the risk of stroke and atherosclerosis with fruit intake | Fruits and vegetables reduce risk of stroke in individuals with PON1 rs662 polymorphism |
| Woo et al. 2018 [77] | Dietary estimation through self-reported FFQ | Cross sectional analysis of 4000 men and women aged 65 years and over in Hong Kong, China | High score on fruit and vegetable intake or Mediterranean diet decreased ABI | Fruit and vegetable consumption is associated with better cardiovascular health |
| Carnevale et al. 2018 [78] | 0.2 mg/mL extra virgin olive oil (EVOO) with or without 500 U/mL catalase and stimula-ted for 10 min with 0.5 mM arachidonic acid | Blood platelets taken from 5 healthy subjects (3 male, 2 female, mean age 39.8 years) | Both catalase and EVOOs reduced the activation of Nox2 and H2O2 production | EVOO reduced oxidative stress by decreasing H2O2 levels and Nox2 activity |
| Farras et al. 2018 [79] | 3-week consumption of 25 mL/day virgin olive oil (VOO), plus its own polyphenols (FVOO), or plus thyme polyphenols (FVOOT) | 33 hypercholesteremic individuals | FVOO and FVOOT increased HDL, while FVOOT increased α-tocopherol | Olive oil enriched in polyphenols increases HDL levels and antioxidant capacity |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2019, 11, 53. https://doi.org/10.3390/nu11010053
Serino A, Salazar G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients. 2019; 11(1):53. https://doi.org/10.3390/nu11010053
Chicago/Turabian StyleSerino, Alexa, and Gloria Salazar. 2019. "Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease" Nutrients 11, no. 1: 53. https://doi.org/10.3390/nu11010053