Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Bone Marrow-Derived Macrophages
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Oral Administration and Sample Collection
2.5. RNA Preparation and Quantitative RT-PCR from Tissues
2.6. Light Exposure
2.7. Retinal Cell Preparations
2.8. Flow Cytometry Analyses
2.9. Analysis of Cytokine Concentrations
2.10. Measurements of the Retinal Thickness
2.11. Electroretinography (ERG)
2.12. Statistical Analysis
3. Results
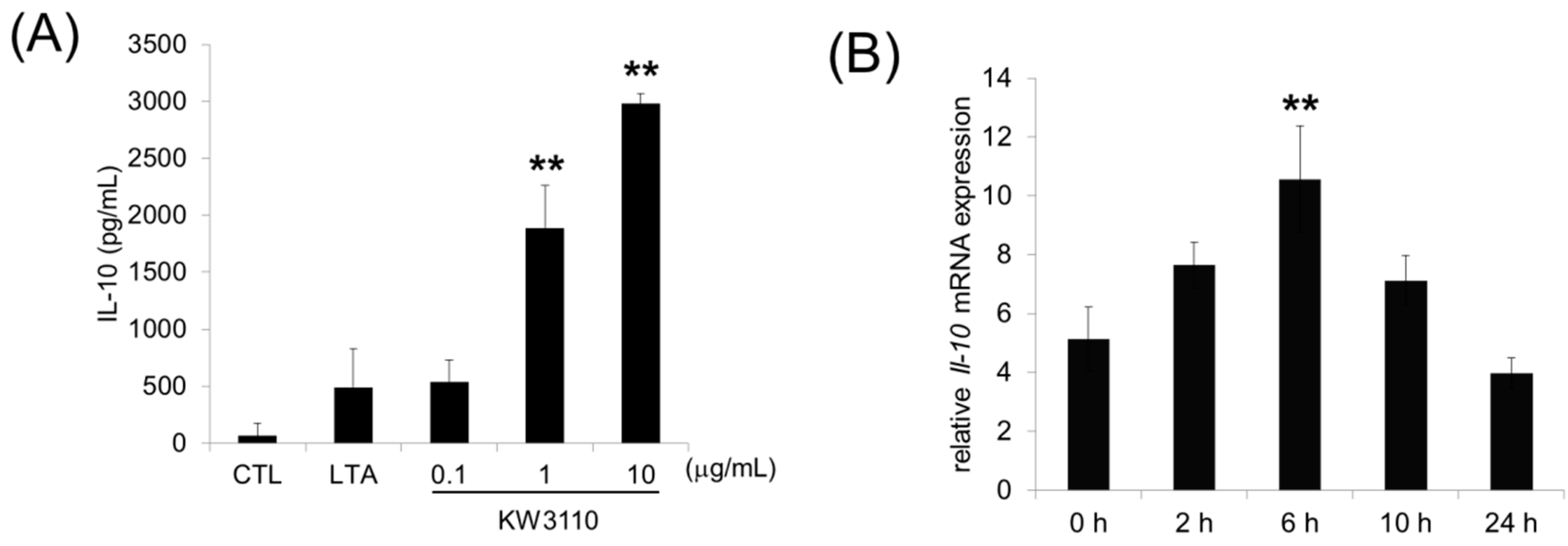
3.1. L. paracasei KW3110 Activates M2 Macrophages In Vitro and Induces IL-10 Production In Vivo
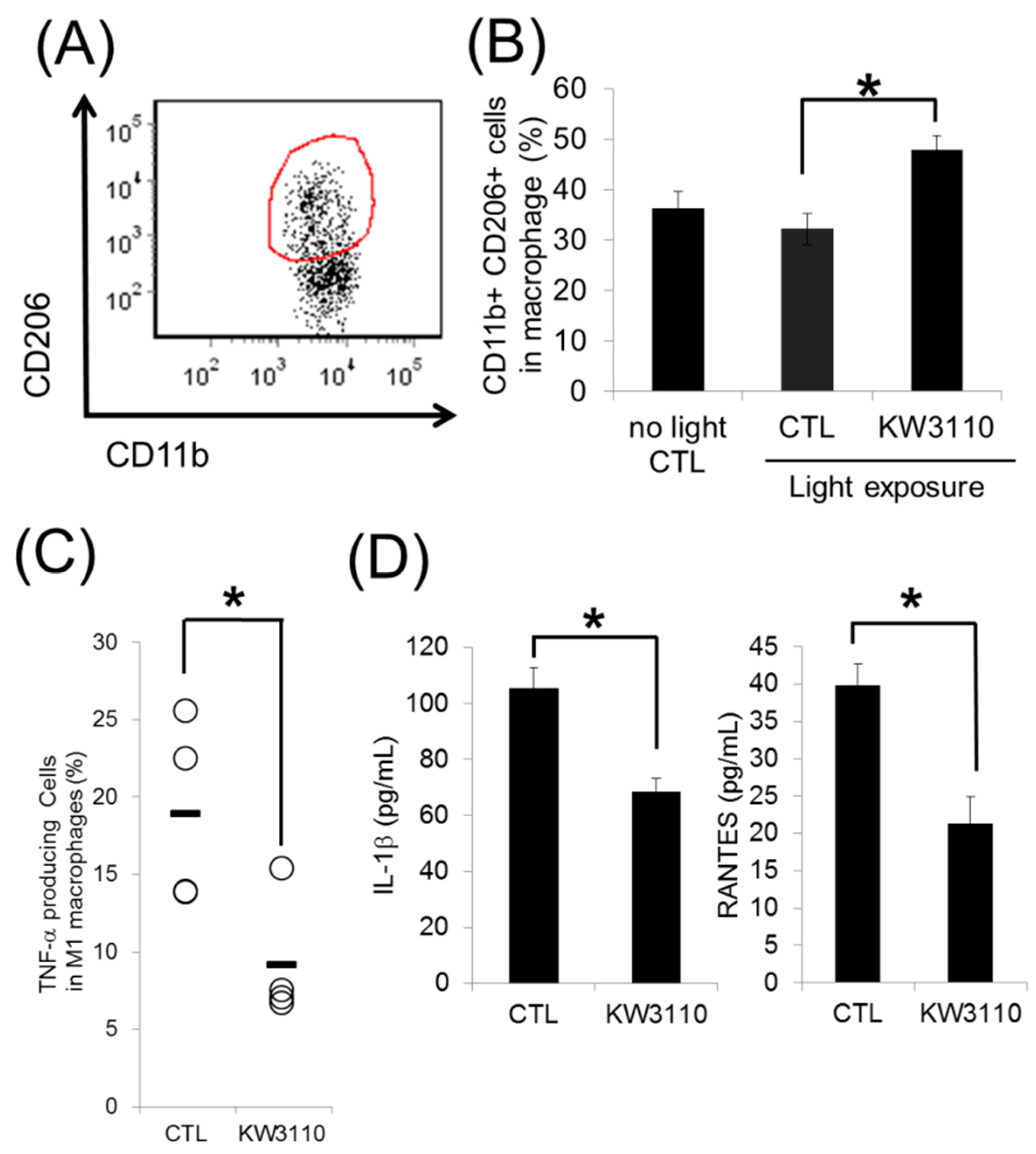
3.2. L. paracasei KW3110 Induces Retinal M2 Macrophages Following Light Exposure
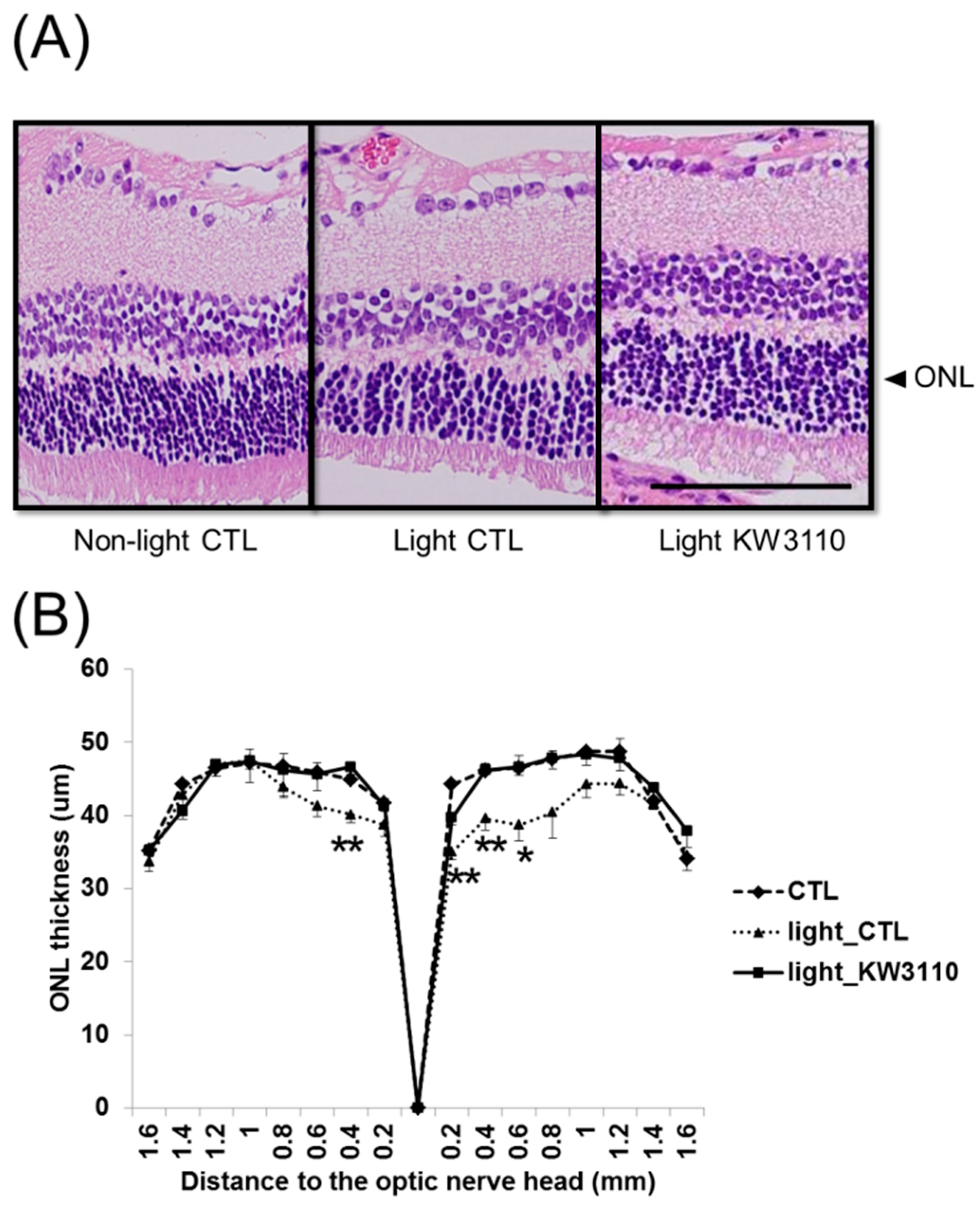
3.3. Intake of L. paracasei KW3110 Suppresses the Photoreceptor Degeneration Induced by Light Exposure
3.4. Intake of L. paracasei KW3110 Attenuates the Impairment of Retinal Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kishi, S.; Li, D.; Takahashi, M.; Hashimoto, H. Photoreceptor damage after prolonged gazing at a computer game display. Jpn. J. Ophthalmol. 2010, 54, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tsujikawa, M.; Itabe, H.; Du, Z.J.; Xie, P.; Matsumura, N.; Fu, X.; Zhang, R.; Sonoda, K.H.; Egashira, K.; et al. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J. Cell Sci. 2012, 125, 2407–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer-Hockstein, C.; Dunaief, J.L. Could blue light-blocking lenses decrease the risk of age-related macular degeneration? Retina 2006, 26, 1–4. [Google Scholar] [CrossRef]
- Reme, C.E.; Grimm, C.; Hafezi, F.; Marti, A.; Wenzel, A. Apoptotic cell death in retinal degenerations. Prog. Retin. Eye Res. 1998, 17, 443–464. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, 106–116. [Google Scholar] [CrossRef]
- Choi, W.; Kim, J.C.; Kim, W.S.; Oh, H.J.; Yang, J.M.; Lee, J.B.; Yoon, K.C. Clinical Effect of Antioxidant Glasses Containing Extracts of Medicinal Plants in Patients with Dry Eye Disease: A Multi-Center, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2015, 10, e0139761. [Google Scholar] [CrossRef]
- Uchino, Y.; Uchino, M.; Dogru, M.; Fukagawa, K.; Tsubota, K. Improvement of accommodation with anti-oxidant supplementation in visual display terminal users. J. Nutr. Health Aging 2012, 16, 478–481. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal Protection and Distribution of Curcumin in Vitro and in Vivo. Front. Pharmacol. 2018, 9, 670. [Google Scholar] [CrossRef]
- Kurihara, T.; Westenskow, P.D.; Gantner, M.L.; Usui, Y.; Schultz, A.; Bravo, S.; Aguilar, E.; Wittgrove, C.; Friedlander, M.; Paris, L.P.; et al. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife 2016, 5, e14319. [Google Scholar] [CrossRef]
- Jiao, H.; Natoli, R.; Valter, K.; Provis, J.M.; Rutar, M. Spatiotemporal Cadence of Macrophage Polarisation in a Model of Light-Induced Retinal Degeneration. PLoS ONE 2015, 10, e0143952. [Google Scholar] [CrossRef]
- Rutar, M.; Natoli, R.; Provis, J.M. Small interfering RNA-mediated suppression of Ccl2 in Muller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J. Neuroinflamm. 2012, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Noailles, A.; Fernandez-Sanchez, L.; Lax, P.; Cuenca, N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J. Neuroinflammation 2014, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Satoh, T.; Kidoya, H.; Naito, H.; Yamamoto, M.; Takemura, N.; Nakagawa, K.; Yoshioka, Y.; Morii, E.; Takakura, N.; Takeuchi, O.; et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature 2013, 495, 524–528. [Google Scholar] [CrossRef]
- London, A.; Itskovich, E.; Benhar, I.; Kalchenko, V.; Mack, M.; Jung, S.; Schwartz, M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med. 2011, 208, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Kubota, S.; Kurihara, T.; Ebinuma, M.; Kubota, M.; Yuki, K.; Sasaki, M.; Noda, K.; Ozawa, Y.; Oike, Y.; Ishida, S.; et al. Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. Am. J. Pathol. 2010, 177, 1725–1731. [Google Scholar] [CrossRef]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef]
- Ichikawa, S.; Miyake, M.; Fujii, R.; Konishi, Y. Orally administered Lactobacillus paracasei KW3110 induces in vivo IL-12 production. Biosci. Biotechnol. Biochem. 2009, 73, 1561–1565. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Nariai, C.; Takemura, F.; Nakao, W.; Fujiwara, D. Dietary supplementation with lactic acid bacteria attenuates the development of atopic-dermatitis-like skin lesions in NC/Nga mice in a strain-dependent manner. Int. Arch. Allergy Immunol. 2008, 145, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Inoue, S.; Wakabayashi, H.; Fujii, T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int. Arch. Allergy Immunol. 2004, 135, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Wakabayashi, H.; Watanabe, H.; Nishida, S.; Iino, H. A Double-blind Trial of Lactobacillus paracasei Strain KW3110 Administration for Immunomodulation in Patients with Pollen Allergy. Allergol. Int. 2005, 54, 143–149. [Google Scholar] [CrossRef]
- Morita, Y.; Jounai, K.; Miyake, M.; Inaba, M.; Kanauchi, O. Effect of Heat-Killed Lactobacillus paracasei KW3110 Ingestion on Ocular Disorders Caused by Visual Display Terminal (VDT) Loads: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Study. Nutrients 2018, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, A.J.; Lawrence, T.; Hamilton, J.A.; Cook, A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. J. Immunol. 2007, 178, 5245–5252. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, A.J.; Dinh, H.; Cook, A.D.; Hertzog, P.J.; Hamilton, J.A. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J. Leukoc. Biol. 2009, 86, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Kaji, R.; Kiyoshima-Shibata, J.; Nagaoka, M.; Nanno, M.; Shida, K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J. Immunol. 2010, 184, 3505–3513. [Google Scholar] [CrossRef]
- Macho Fernandez, E.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Inagawa, H.; Kohchi, C.; Kazumura, K.; Tsuchiya, H.; Miwa, T.; Okazaki, K.; Soma, G.I. Oral administration of Pantoea agglomerans-derived lipopolysaccharide prevents metabolic dysfunction and Alzheimer’s disease-related memory loss in senescence-accelerated prone 8 (SAMP8) mice fed a high-fat diet. PLoS ONE 2018, 13, e0198493. [Google Scholar] [CrossRef]
- Mia, S.; Warnecke, A.; Zhang, X.M.; Malmstrom, V.; Harris, R.A. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-beta yields a dominant immunosuppressive phenotype. Scand. J. Immunol. 2014, 79, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Francke, M.; Ulbricht, E.; Beck, S.; Seeliger, M.; Hirrlinger, P.; Hirrlinger, J.; Lang, K.S.; Zinkernagel, M.; Odermatt, B.; et al. Cooperative phagocytes: Resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions. Am. J. Pathol. 2009, 174, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuse, Y.; Tsuruma, K.; Kanno, Y.; Shimazawa, M.; Hara, H. CCR3 Is Associated with the Death of a Photoreceptor Cell-line Induced by Light Exposure. Front. Pharmacol. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.S.; McLaughlin, W.M.; Vasilakes, N.; Echevarria, F.D.; Formichella, C.R.; Sappington, R.M. Constitutive and Stress-induced Expression of CCL5 Machinery in Rodent Retina. J. Clin. Cell Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Steinbach, J.P.; Marti, A.; Munz, K.; Wang, Z.Q.; Wagner, E.F.; Aguzzi, A.; Reme, C.E. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat. Med. 1997, 3, 346–349. [Google Scholar] [CrossRef]
- Lei, B.; Yao, G.; Zhang, K.; Hofeldt, K.J.; Chang, B. Study of rod- and cone-driven oscillatory potentials in mice. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2732–2738. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Yuki, K.; Nagai, N.; Tsubota, K. Biological effects of blocking blue and other visible light on the mouse retina. Clin. Exp. Ophthalmol. 2014, 42, 555–563. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Anderson, R.E. Protective effects of soft acrylic yellow filter against blue light-induced retinal damage in rats. Exp. Eye Res. 2006, 83, 1493–1504. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Anderson, R.E. Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Saenz-de-Viteri, M.; Heras-Mulero, H.; Fernandez-Robredo, P.; Recalde, S.; Hernandez, M.; Reiter, N.; Moreno-Orduna, M.; Garcia-Layana, A. Oxidative stress and histological changes in a model of retinal phototoxicity in rabbits. Oxid. Med. Cell Longev. 2014, 2014, 637137. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Parish, C.A.; Hashimoto, M.; Nakanishi, K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2988–2995. [Google Scholar] [PubMed]
- Marshall, J.; Mellerio, J.; Palmer, D.A. Damage to pigeon retinae by moderate illumination from fluorescent lamps. Exp. Eye Res. 1972, 14, 164–169. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, Y.; Miwa, Y.; Jounai, K.; Fujiwara, D.; Kurihara, T.; Kanauchi, O. Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina. Nutrients 2018, 10, 1991. https://doi.org/10.3390/nu10121991
Morita Y, Miwa Y, Jounai K, Fujiwara D, Kurihara T, Kanauchi O. Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina. Nutrients. 2018; 10(12):1991. https://doi.org/10.3390/nu10121991
Chicago/Turabian StyleMorita, Yuji, Yukihiro Miwa, Kenta Jounai, Daisuke Fujiwara, Toshihide Kurihara, and Osamu Kanauchi. 2018. "Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina" Nutrients 10, no. 12: 1991. https://doi.org/10.3390/nu10121991




