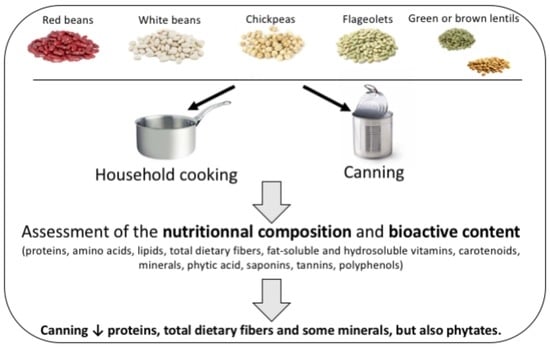
Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Household Cooking Method
2.2.2. Canning Method
2.2.3. Stabilization Method
2.3. Nutritional Composition Analysis of Pulses
2.3.1. Protein and Amino Acid Composition
2.3.2. Lipid Composition
2.3.3. Fat-Soluble Micronutrient Composition
2.3.4. Analysis of Bioactive Compounds
2.3.5. Total Dietary Fiber, Water-Soluble Vitamin and Mineral Contents
2.4. Statistical Analysis
3. Results
3.1. Proteins
3.2. Lipids
3.3. Total Dietary Fibers (TDF)
3.4. Fat-Soluble Micronutrients
3.5. Water-Soluble Vitamins
3.6. Minerals
3.7. Bioactive Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaudhary, A.; Marinangeli, C.P.F.; Tremorin, D.; Mathys, A. Nutritional combined greenhouse gas life cycle analysis for incorporating canadian yellow pea into cereal-based food products. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Hamel, C.; O’Donovan, J.T.; Cutforth, H.; Zentner, R.P.; Campbell, C.A.; Niu, Y.; Poppy, L. Diversifying crop rotations with pulses enhances system productivity. Sci. Rep. 2015, 5, 14625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leterme, P. Recommendations by health organizations for pulse consumption. Br. J. Nutr. 2002, 88, S239–S242. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Major, A.W. Pulses and lipaemia, short- and long-term effect: Potential in the prevention of cardiovascular disease. Br. J. Nutr. 2002, 88 (Suppl. 3), S263–S271. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Non-soy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial effects of common bean on adiposity and lipid metabolism. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Fechner, A.; Fenske, K.; Jahreis, G. Effects of legume kernel fibres and citrus fibre on putative risk factors for colorectal cancer: A randomised, double-blind, crossover human intervention trial. Nutr. J. 2013, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.M. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 2002, 88 Suppl 3, S307–S319. [Google Scholar] [CrossRef]
- Harland, B.F.; Morris, E.R. Phytate—A Good or a Bad Food Component. Nutr. Res. 1995, 15, 733–754. [Google Scholar] [CrossRef]
- Jenkins, K.J.; Atwal, A.S. Effects of dietary saponins on fecal bile acids and neutral sterols, and availability of vitamins A and E in the chick. J. Nutr. Biochem. 1994, 5, 134–137. [Google Scholar] [CrossRef]
- Johnson, I.T.; Gee, J.M.; Price, K.; Curl, C.; Fenwick, G.R. Influence of saponins on gut permeability and active nutrient transport in vitro. J. Nutr. 1986, 116, 2270–2277. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.; Mateus, N.; Pianet, I.; Laguerre, M.; de Freitas, V. Mechanisms of tannin-induced trypsin inhibition: a molecular approach. Langmuir 2011, 27, 13122–13129. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.G.; Goncalves, G.A.; Peralta, R.A.; Seixas, F.A.V.; de Sa-Nakanishi, A.B.; Bracht, L.; Comar, J.F.; Bracht, A.; Peralta, R.M. Inhibition of alpha-amylases by condensed and hydrolysable tannins: Focus on kinetics and hypoglycemic actions. Enzyme Res. 2017, 2017, 5724902. [Google Scholar] [CrossRef] [PubMed]
- Helsper, J.P.F.G.; Hoogendijk, J.M.; van Norel, A.; Burger-Meyer, K. Antinutritional factors in faba beans (Vica faba L.) as affected by breeding toward the absence of condensed tannins. J. Agric. Food Chem. 1993, 41, 1058–1061. [Google Scholar] [CrossRef]
- Lestienne, I.; Besancon, P.; Caporiccio, B.; Lullien-Pellerin, V.; Treche, S. Iron and zinc in vitro availability in pearl millet flours (Pennisetum glaucum) with varying phytate, tannin, and fiber contents. J. Agric. Food Chem. 2005, 53, 3240–3247. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Gaxiola, S.; Chuck-Hernandez, C.; Serna Saldivar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Salunke, D.K.; Cheryan, M. Technology of removal of unwanted components of dry beans. Crit. Rev. Food Sci. Nutr. 1984, 21, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Gleize, B.; Steib, M.; Andre, M.; Reboul, E. Simple and fast HPLC method for simultaneous determination of retinol, tocopherols, coenzyme Q(10) and carotenoids in complex samples. Food Chem. 2012, 134, 2560–2564. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Margier, M.; Roi, S.; Collet, X.; Niot, I.; Goupy, P.; Caris-Veyrat, C.; Reboul, E. Intestinal scavenger receptors are involved in vitamin K1 absorption. J. Biol. Chem. 2014, 289, 30743–30752. [Google Scholar] [CrossRef] [PubMed]
- Dost, K.; Tokul, O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal. Chim. Acta 2006, 558, 22–27. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Lásztity, R.; Hidvegi, M. Amino Acid Composition and Biological Value of Cereal Proteins (Proceedings of the International Association for Cereal Chemistry Symposium on Amino Acid Composition and Biological Value of Cereal Proteins); D. Reidel Publishing Compagny: Budapest, Hungaria, 1985. [Google Scholar]
- AFSSA. Apport en protéines: Consommation, qualité, besoins et recommandations; AFSSA: Maison Alfort, France, 2007. [Google Scholar]
- Martin-Cabrejas, M.A.; Aguilera, Y.; Pedrosa, M.M.; Cuadrado, C.; Hernandez, T.; Diaz, S.; Esteban, R.M. The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem. 2009, 114, 1063–1068. [Google Scholar] [CrossRef]
- Table de composition nutritionnelle des aliments Ciqual. 2017. Available online: https://ciqual.anses.fr/ (accessed on 1 November 2018).
- Reboul, E. Vitamin E bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants (Basel) 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Scripsema, N.K.; Hu, D.N.; Rosen, R.B. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J. Ophthalmol. 2015, 2015, 865179. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Lloris, F.; Gimenez, R.; Olalla, M.; Lopez, M.C. Mineral content in legumes and nuts: Contribution to the Spanish dietary intake. Sci. Total Environ. 2003, 308, 1–14. [Google Scholar] [CrossRef]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. NMCD 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Effect of processing on nutritive value of food: canning (Fruit, juices, vegetables, meat, fish). In Handbook of Nutritive Value of Processed Food; Rechcígl, M. (Ed.) CRC Press: Boca Raton, FL, USA, 1982. [Google Scholar]
- Rickman, J.C.; Bruhn, C.M.; Barrett, D.M. Nutritional comparison of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. J. Sci. Food Agric. 2007, 87, 1185–1196. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hatcher, D.W.; Tyler, R.T.; Toews, R.; Gawalko, E.J. Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Siddhuraju, P.; Pugalenthi, M.; Janardhanan, K. Effect of soaking and heat processing on the levels of antinutrients and digestible proteins in seeds of Vigna aconitifolia and Vigna sinensis. Food Chem. 1998, 63, 259–264. [Google Scholar] [CrossRef]
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.M.; Martin-Cabrejas, M.A. Bioactive phenolic compounds and functional properties of dehydrated bean flours. Food Res. Int. 2011, 44, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Livny, O.; Reifen, R.; Levy, I.; Madar, Z.; Faulks, R.; Southon, S.; Schwartz, B. Beta-carotene bioavailability from differently processed carrot meals in human ileostomy volunteers. Eur. J. Nutr. 2003, 42, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.M.; Winham, D.M.; Oberhauser, A.M.; Litchfield, R.E. Socio-ecological barriers to dry grain pulse consumption among low-income women: A mixed methods approach. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Masset, G.; Soler, L.G.; Vieux, F.; Darmon, N. Identifying sustainable foods: The relationship between environmental impact, nutritional quality, and prices of foods representative of the French diet. J. Acad. Nutr. Diet. 2014, 114, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
| Pulses | Kidney Beans | White Beans | Chickpeas | Green Lentils | Brown Lentils | Flageolets | |||
|---|---|---|---|---|---|---|---|---|---|
| Cooking Method | Household Cooking | Canning | Household Cooking | Canning | Household Cooking | Canning | Household Cooking | Canning | Household Cooking |
| Proteins (g/100 g) | 9.67 ± 0.10 a | 6.74 ± 0.14 f,* | 7.20 ± 0.08 b | 5.92 ± 0.12 g,* | 7.82 ± 0.08 c | 6.46 ± 0.13 h,* | 8.31 ± 0.09 d | 5.08 ± 0.10 i | 6.92 ± 0.07 e |
| Essential AA | 4.47 | 3.18 | 3.42 | 2.51 | 3.45 | 2.89 | 3.43 | 2.15 | 3.33 |
| Non-essential AA | 5.20 | 3.56 | 3.79 | 3.41 | 4.37 | 3.57 | 4.88 | 2.93 | 3.59 |
| Lipids | |||||||||
| Total lipids (g/100 g) | 0.28 ± 0.03 a | 0.29 ± 0.01 f | 0.26 ± 0.01 a | 0.23 ± 0.03 g | 2.04 ± 0.03 b | 1.78 ± 0.06 h,* | 0.29 ± 0.01 a | 0.19 ± 0.01 i | 0.51 ± 0.03 c |
| Triglycerides (g/100 g) | 0.16 ± 0.01 a | 0.20 ± 0.01 f,* | 0.21 ± 0.01 b | 0.19 ± 0.03 f | 1.92 ± 0.00 c | 1.69 ± 0.03 g,* | 0.16 ± 0.02 a | 0.14 ± 0.00 h | 0.44 ± 0.04 d |
| Phospholipids (mg/100 g) | 20.84 ± 0.41 a | 20.94 ± 2.53 f | 17.98 ± 0.95 b | 11.97 ± 3.36 g,* | 165.85 ± 0.65 c | 156.09 ± 5.38 h,* | 23.96 ± 0.76 d | 17.90 ± 1.44 f | 39.31 ± 4.28 e |
| Sterols (mg/100 g) | 22.05 ± 1.23 a | 29.62 ± 1.23 f,* | 29.21 ± 0.77 b | 28.25 ± 2.88 f | 114.99 ± 12.88 c | 121.02 ± 9.26 g | 29.15 ± 3.93 b | 19.16 ± 0.30 h | 43.25 ± 0.81 d |
| Total dietary fibers (g/100 g) | 11.60 ± 2.50 ab | 6.50 ± 2.10 f,* | 10.00 ± 2.40 ab | 6.70 ± 2.20 f | 8.20 ± 2.30 a | 6.40 ± 2.10 f | 8.50 ± 2.30 a | 3.80 ± 1.90 f | 13.80 ± 2.70 a |
| Fat-soluble micronutrients | |||||||||
| γ-tocopherol (mg/100 g) | 0.43 ± 0.21 a | 1.02 ± 0.23 f,* | n.d. | n.d. | 2.20 ± 0.40 b | 2.88 ± 0.64 g | 4.43 ± 0.40 c | 3.51 ± 0.63 g | 0.27 ± 0.10 a |
| β-carotene (µg/100 g) | n.d. | n.d. | n.d. | n.d. | 14.56 ± 2.95 a | 14.69 ± 2.59 f | 14.34 ± 1.91 a | 6.87 ± 0.71 g | n.d. |
| Lutein (µg/100 g) | 4.62 ± 1.02 a | 5.65 ± 0.61 f | n.d. | n.d. | 106.65 ± 53.58 b | 185.86 ± 62.76 g | 748.76 ± 76.03 c | 818.90 ± 110.02 h | 7.48 ± 1.84 d |
| Zeaxanthin (µg/100 g) | 0.78 ± 0.18 a | 0.86 ± 0.10 f | n.d. | n.d. | 10.19 ± 5.12 b | 11.21 ± 1.35 g | 45.89 ± 4.92 c | 49.41 ± 6.76 h | n.d. |
| Water-soluble vitamins (µg/100 g) | |||||||||
| B1 | 117.00 ± 19.00 a | 90.00 ± 14.40 f | 23.00 ± 3.70 b | 20.00 ± 3.20 g | 60.00 ± 9.60 c | 50.00 ± 8.00 h | 72.00 ± 11.50 c | 40.00 ± 6.40 i | 30.00 ± 4.80 b |
| B2 | 220.00 ± 3.50 a | n.d. | 15.80 ± 2.50 b | n.d. | 26.80 ± 4.30 c | n.d. | 32.70 ± 5.20 c | n.d. | 16.80 ± 2.70 b |
| B5 | 83.30 ± 20.00 a | 90.40 ± 21.70 fg | 72.70 ± 17.40 a | 74.40 ± 17.90 g | 138.00 ± 33.00 b | 146.00 ± 35.00 fh | 318.00 ± 76.00 c | 151.00 ± 36.00 h | 60.70 ± 41.60 a |
| B6 | 67.00 ± 9.40 a | 58.20 ± 8.10 fg | 48.20 ± 6.70 b | 56.10 ± 7.90 fg | 90.40 ± 12.70 c | 71.40 ± 10.00 f | 134.00 ± 19.00 d | 53.60 ± 7.50 g | 39.00 ± 5.50 b |
| B9 | 19.49 ± 3.40 a | 21.23 ± 2.54 f | 22.25 ± 0.31 a | 9.36 ± 0.63 g,* | 67.62 ± 7.78 b | 34.30 ± 0.92 h,* | 26.02 ± 5.12 a | 21.08 ± 1.30 f | 26.31 ± 4.18 a |
| Minerals (mg/100 g) | |||||||||
| Calcium | 55.00 ± 11.00 a | 43.00 ± 8.60 f | 79.00 ± 16.00 a | 77.00 ± 15.00 g | 72.00 ± 14.00 a | 52.00 ± 10.00 f | 33.00 ± 6.60 b | 27.00 ± 5.40 h | 120.00 ± 24.00 c |
| Copper | 0.27 ± 0.05 a | 0.29 ± 0.06 f | 0.22 ± 0.04 a | 0.26 ± 0.05 f | 0.24 ± 0.05 a | 0.24 ± 0.06 f | 0.21 ± 0.04 a | 0.18 ± 0.04 f | 0.19 ± 0.04 a |
| Iron | 2.30 ± 0.46 a | 1.80 ± 0.04 f,* | 1.60 ± 0.32 a | 1.50 ± 0.30 f | 1.30 ± 0.26 a | 1.30 ± 0.26 f | 2.00 ± 0.40 a | 1.50 ± 0.30 f | 1.70 ± 0.34 a |
| Magnesium | 39.00 ± 7.80 a | 26.00 ± 5.20 f,* | 37.00 ± 7.40 a | 31.00 ± 6.20 f | 44.00 ± 8.80 a | 28.00 ± 5.60 f,* | 32.00 ± 6.40 a | 13.00 ± 2.60 f | 33.00 ± 6.60 a |
| Manganese | 0.44 ± 0.09 a | 0.33 ± 0.07 f | 0.55 ± 0.11 ab | 0.50 ± 0.10 g | 0.86 ± 0.17 c | 0.67 ± 0.13 g | 0.38 ± 0.08 a | 0.26 ± 0.05 f | 0.80 ± 0.16 bc |
| Phosphorus | 150.00 ± 30.00 a | 110.00 ± 22.00 f | 120.00 ± 24.00 a | 110.00 ± 22.00 f | 140.00 ± 28.00 a | 97.00 ± 19.00 f | 140.00 ± 28.00 a | 53.00 ± 11.00 f | 110.00 ± 22.00 a |
| Potassium | 300.00 ± 60.00 a | 250.00 ± 50.00 f | 230.00 ± 46.00 ab | 230.00 ± 46.00 f | 170.00 ± 34.00 b | 140.00 ± 28.00 g | 230.00 ± 46.00 ab | 81.00 ± 16.00 h | 260.00 ± 52.00 a |
| Zinc | 0.94 ± 0.19 a | 0.77 ± 0.16 f | 0.76 ± 0.16 a | 0.74 ± 0.15 f | 1.10 ± 0.22 a | 1.10 ± 0.22 f | 0.99 ± 0.20 a | 0.48 ± 0.10 g | 0.67 ± 0.14 a |
| Bioactive/Antinutritional compounds (mg/100 g) | |||||||||
| Phytic acid | 627.33 ± 42.35 ad | 386.32 ± 25.46 f,* | 532.43 ± 28.28 b | 470.31 ± 48.05 gh | 693.94 ± 40.67 acd | 526.40 ± 22.26 g,* | 714.46 ± 28.85 cd | 412.59 ± 36.26 fh | 683.66 ± 65.66 d |
| Saponins | 106.02 ± 4.11 a | 118.60 ± 4.84 f | 103.95 ± 5.69 a | 107.58 ± 0.55 g | 121.86 ± 4.68 b | 116.84 ± 0.22 f,* | 174.73 ± 2.66 c | 98.73 ± 2.72 h | 122.12 ± 2.33 b |
| Tannins | 6.78 ± 0.36 a | 5.44 ± 0.27 f,* | 1.69 ± 0.04 b | 1.82 ± 0.11 g | 16.61 ± 1.89 c | 13.67 ± 1.48 h | 12.53 ± 0.18 d | 7.54 ± 0.08 i | 2.24 ± 0.10 e |
| Total polyphenols | 66.34 ± 2.45 a | 67.17 ± 2.48 f | 15.00 ± 1.08 b | 18.66 ± 1.34 g | 24.98 ± 1.15 c | 27.59 ± 1.27 h | 284.32 ± 3.26 d | 71.84 ± 0.82 i | 15.77 ± 0.75 b |
| Dry matter (g/100 g) | 34.05 | 31.00 | 30.02 | 28.20 | 38.09 | 33.80 | 34.28 | 24.20 | 33.58 |
| Pulses | Red Beans | White Beans | Chickpeas | Green Lentils | Brown Lentils | Flageolets | |||
|---|---|---|---|---|---|---|---|---|---|
| Cooking Method | Household cooking | Canning | Household cooking | Canning | Household cooking | Canning | Household cooking | Canning | Household cooking |
| Essential AA (g/100g) | |||||||||
| Cys | 0.095 ± 0.008 ab | 0.090 ± 0.016 f | 0.087 ± 0.008 ab | 0.083 ± 0.014 f | 0.081 ± 0.007 bc | 0.075 ± 0.013 f | 0.064 ± 0.006 c | 0.072 ± 0.012 f | 0.114 ± 0.010 ab |
| His | 0.279 ± 0.004 a | 0.184 ± 0.005 f,* | 0.203 ± 0.003 b | 0.151 ± 0.004 g,* | 0.214 ± 0.003 bc | 0.177 ± 0.005 f,* | 0.222 ± 0.003 c | 0.129 ± 0.004 h | 0.190 ± 0.003 d |
| Ile | 0.458 ± 0.008 a | 0.346 ± 0.012 f,* | 0.363 ± 0.006 bc | 0.234 ± 0.008 g,* | 0.379 ± 0.007 b | 0.307 ± 0.011 h,* | 0.355 ± 0.006 c | 0.201 ± 0.007 i | 0.333 ± 0.006 d |
| Leu | 0.785 ± 0.007 a | 0.589 ± 0.011 f,* | 0.632 ± 0.006 b | 0.459 ± 0.009 g,* | 0.635 ± 0.006 b | 0.535 ± 0.010 h,* | 0.620 ± 0.006 b | 0.394 ± 0.007 i | 0.594 ± 0.006 c |
| Lys | 0.670± 0.011 a | 0.437 ± 0.005 f,* | 0.487 ± 0.008 b | 0.412 ± 0.005 g,* | 0.521 ± 0.008 c | 0.426 ± 0.005 fg,* | 0.576 ± 0.009 d | 0.354 ± 0.004 h | 0.475 ± 0.008 b |
| Met | 0.105 ± 0.014 a | 0.067 ± 0.013 f,* | 0.086 ± 0.012 b | 0.059 ± 0.011 f,* | 0.087 ± 0.012 b | 0.075 ± 0.014 f | 0.040 ± 0.005 c | 0.051 ± 0.010 f | 0.098 ± 0.013 ab |
| Phe | 0.572 ± 0.013 a | 0.400 ± 0.008 f,* | 0.429 ± 0.010 bd | 0.320 ± 0.006 g,* | 0.476 ± 0.011 c | 0.414 ± 0.008 f,* | 0.441 ± 0.010 b | 0.275 ± 0.005 h | 0.410 ± 0.009 d |
| Thr | 0.457 ± 0.006 a | 0.316 ± 0.003 f,* | 0.340 ± 0.005 b | 0.253 ± 0.003 g,* | 0.364 ± 0.005 c | 0.299 ± 0.003 h,* | 0.349 ± 0.005 b | 0.217 ± 0.002 i | 0.348 ± 0.005 b |
| Trp | 0.110 ± 0.034 ac | 0.071 ± 0.020 f | 0.066 ± 0.020 abc | 0.061 ± 0.017 f | 0.037 ± 0.011 b | 0.041 ± 0.012 f | 0.064 ± 0.019 c | 0.052 ± 0.015 f | 0.086 ± 0.026 c |
| Tyr | 0.425 ± 0.009 a | 0.295 ± 0.007 f,* | 0.314 ± 0.007 b | 0.215 ± 0.005 g,* | 0.280 ± 0.006 c | 0.241 ± 0.005 h,* | 0.301 ± 0.007 bc | 0.185 ± 0.004 i | 0.298 ± 0.006 c |
| Val | 0.512 ± 0.002 a | 0.384 ± 0.010 f,* | 0.407 ± 0.002 b | 0.263 ± 0.007 g,* | 0.379 ± 0.002 c | 0.303 ± 0.008 h,* | 0.340 ± 0.002 d | 0.226 ± 0.006 i | 0.387 ± 0.002 e |
| Total essential AA | 4.469 | 3.180 | 3.415 | 2.509 | 3.453 | 2.891 | 3.429 | 2.153 | 3.332 |
| Non-essential AA (g/100g) | |||||||||
| Ala | 0.409 ± 0.003 a | 0.280 ± 0.006 fg,* | 0.305 ± 0.002 b | 0.262 ± 0.006 f,* | 0.350 ± 0.003 c | 0.286 ± 0.006 g,* | 0.363 ± 0.003 d | 0.225 ± 0.005 h | 0.300 ± 0.002 b |
| Arg | 0.581 ± 0.015 a | 0.375 ± 0.011 f,* | 0.403 ± 0.010 b | 0.477 ± 0.014 g,* | 0.629 ± 0.016 c | 0.514 ± 0.015 h,* | 0.721 ± 0.018 d | 0.409 ± 0.012 i | 0.391 ± 0.010 e |
| Asp ¤ | 1.170± 0.014 a | 0.807 ± 0.015 f,* | 0.858 ± 0.010 b | 0.713 ± 0.014 g,* | 0.946 ± 0.011 c | 0.775 ± 0.015 f,* | 1.022 ± 0.012 d | 0.612 ± 0.012 h | 0.822 ± 0.001 e |
| Glu ¤¤ | 1.555 ± 0.018 a | 1.059 ± 0.024 f,* | 1.122 ± 0.013 b | 1.041 ± 0.024 f,* | 1.297 ± 0.015 c | 1.054 ± 0.024 f,* | 1.522 ± 0.018 d | 0.894 ± 0.020 g | 0.996 ± 0.012 e |
| Gly | 0.381 ± 0.003 a | 0.267 ± 0.005 f,* | 0.287 ± 0.002 b | 0.246 ± 0.005 g,* | 0.320 ± 0.003 c | 0.263 ± 0.005 f,* | 0.349 ± 0.003 d | 0.211 ± 0.004 h | 0.290 ± 0.002 b |
| Pro | 0.476 ± 0.013 a | 0.333 ± 0.012 f,* | 0.355 ± 0.010 b | 0.331 ± 0.012 f,* | 0.389 ± 0.011 c | 0.325 ± 0.012 f,* | 0.443 ± 0.012 a | 0.284 ± 0.011 g | 0.350 ± 0.010 b |
| Ser | 0.629 ± 0.003 a | 0.434 ± 0.011 f,* | 0.459 ± 0.002 b | 0.339 ± 0.008 g,* | 0.437 ± 0.002 c | 0.355 ± 0.000 h,* | 0.464 ± 0.002 b | 0.291 ± 0.007 i | 0.440 ± 0.002 c |
| Total non-essential AA | 5.205 | 3.556 | 3.787 | 3.410 | 4.367 | 3.571 | 4.883 | 2.926 | 3.588 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. https://doi.org/10.3390/nu10111668
Margier M, Georgé S, Hafnaoui N, Remond D, Nowicki M, Du Chaffaut L, Amiot M-J, Reboul E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients. 2018; 10(11):1668. https://doi.org/10.3390/nu10111668
Chicago/Turabian StyleMargier, Marielle, Stéphane Georgé, Noureddine Hafnaoui, Didier Remond, Marion Nowicki, Laure Du Chaffaut, Marie-Josèphe Amiot, and Emmanuelle Reboul. 2018. "Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method" Nutrients 10, no. 11: 1668. https://doi.org/10.3390/nu10111668