The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials
Abstract
:1. Introduction
2. Gut Microbiota–Brain Axis in Alzheimer’s Disease
2.1. The Gut Ecosystem: Profile and Immunity
2.2. The Gut Microbiota and Neuroinflammation
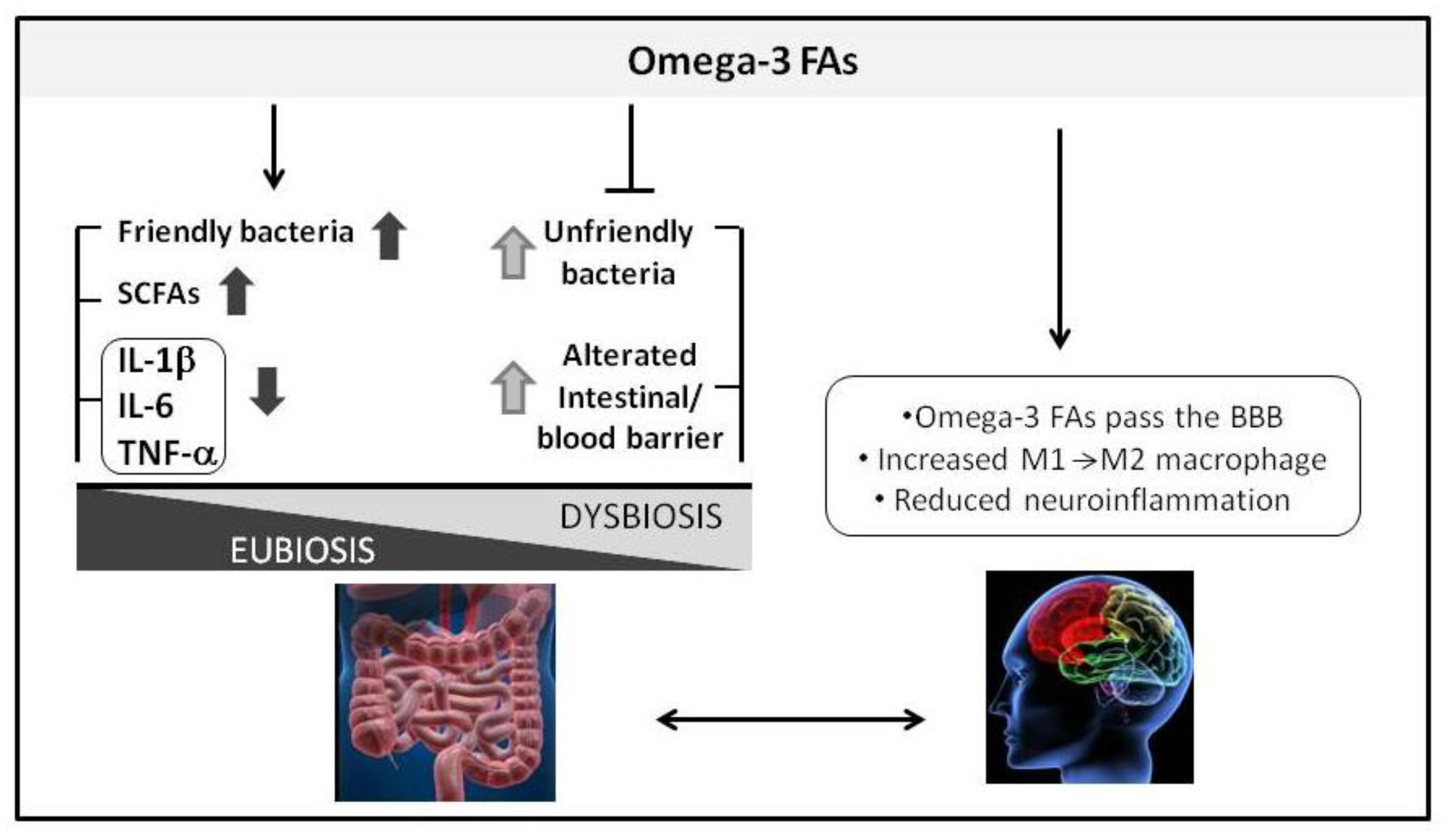
3. Omega-3 Fatty Acids and Gut-Brain Axis in Alzheimer’s Disease
3.1. Omega-3 Fatty Acids and Microbiota
3.2. Omega-3 Fatty Acids: A Nutritional Strategy in Alzheimer’s Disease Treatment?
- It is difficult to establish comparative analysis because of heterogeneity in composition, dosage and duration of supplementation;
- It is difficult to establish comparative analysis because of heterogeneity of methods used to assess cognition (Table 3);
- The placebo group in one study [85] included olive oil, a source of monounsaturated fatty acid. As previously demonstrated by other authors, monounsaturated fatty acids are inversely correlated with cognitive decline and therefore cannot be considered a totally inert substance on cognition performance;
- In all presented studies, the subjects received medications for AD along with omega-3 FAs. This condition could also contribute to good outcomes in control group, hiding and/or slowing down the physiological decline in AD;
- The duration of treatment with omega-3 FAs could be too short for a chronic disease such as AD;
- Omega-3 FAs dosage could be insufficient to provide significant benefits.
4. Probiotic Strategy in Alzheimer’s Disease Treatment?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L. The long-term stability of the human gut microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Biagi, E.; Brigidi, P.; O’Toole, P.W.; De Vos, W.M. Maintenance of a healthy trajectory of the intestinal microbiome during aging: A dietary approach. Mech. Ageing Dev. 2014, 136, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Prat, A. One more role for the gut: Microbiota and blood brain barrier. Ann. Transl. Med. 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein cell. 2016, 8, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Life Science. 2016, 59, 1006–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saresella, M.; Mendozzi, L.; Rossi, V.; Mazzali, F.; Piancone, F.; La Rosa, F.; Marventano, I.; Caputo, D.; Felis, G.E.; Clerici, M. Immunological and Clinical Effect of Diet Modulation of the Gut Microbiome in Multiple Sclerosis Patients: A Pilot Study. Front. Immunol. 2017, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Restrepo, L.; Pellegrini, M. Immunotherapy of Mild Cognitive Impairment by ω-3 Supplementation: Why Are Amyloid-β Antibodies and ω-3 Not Working in Clinical Trials? J. Alzheimers Dis. 2018, 62, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Kooij, G.; Wagner, K.; Hammock, B.; Pellegrini, M. Modulation of innate immunity of patients with Alzheimer’s disease by omega-3 fatty acids. FASEB J. 2017, 31, 3229–3239. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Saresella, M.; Baglio, F.; Piancone, F.; Marventano, I.; Calabrese, E.; Nemni, R.; Ripamonti, E.; Cabinio, M.; Clerici, M. Immune and Imaging Correlates of Mild Cognitive Impairment Conversion to Alzheimer’s Disease. Sci. Rep. 2017, 7, 16760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Rampelli, S.; Soverini, M.; Turroni, S.; Quercia, S.; Biagi, E.; Brigidi, P.; Candela, M. ViromeScan: A new tool for metagenomic viral community profiling. BMC Genomics 2016, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G. Bacterial Metabolism; Springer-Verlag: New York, NY, USA, 1979. [Google Scholar]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.H.; Rombeau, J.L.; Sakata, T. Physiological and Clinical Aspects of Short-Chain Fatty Acids, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: Acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, H.J.; Duncan, S.H.; Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 2017, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Leccioli, V.; Oliveri, M.; Romeo, M.; Berretta, M.; Rossi, P. A New Proposal for the Pathogenic Mechanism of Non-Coeliac/Non-Allergic Gluten/Wheat Sensitivity: Piecing Together the Puzzle of Recent Scientific Evidence. Nutrients 2017, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.M.; Lee, Y.M.; Chang, M.W.; Leblanc, M.; Collins, B.; Downes, M.; Evans, R.M.; Ayres, J.S. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science 2015, 350, 558–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivollier, A.; He, J.; Kole, A.; Valatas, V.; Kelsall, B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012, 209, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cândido, F.G.; Valente, X.F.; Grześkowiak, L.M.; Moreira, A.P.; Rocha, D.M.; Alfenas, R. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2017, 69, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Castagliuolo, I.; Pinzani, M.; Palù, G.; Martines, D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berer, K.; Krishnamoorthy, G. Microbial view of central nervous system autoimmunity. FEBS Lett. 2014, 588, 4207–4213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wekerle, H.; Hohlfeld, R. Gut Microbiota in Multiple Sclerosis: A Bioreactor Driving Brain Autoimmunity. Elsevier 2016, 113–125. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Zárate-Bladés, C.R.; Horai, R.; Caspi, R.R. Regulation of Autoimmunity by the Microbiome. DNA Cell. Biol. 2016, 35, 455–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanisavljević, S.; Lukić, J.; Soković, S.; Mihajlovic, S.; Mostarica Stojković, M.; Miljković, D.; Golić, N. Correlation of Gut Microbiota Composition with Resistance to Experimental Autoimmune Encephalomyelitis in Rats. Front. Microbiol. 2016, 7, 2005. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.P.; Town, T.; Mori, T.; Lue, L.F.; Shytle, D.; Sanberg, P.R.; Morgan, D.; Fernandez, F.; Flavell, R.A.; Tan, J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur. J. Immunol. 2005, 35, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Nikolic, V.; Tan, J. The microglial “activation” continuum: From innate to adaptive responses. J. Neuroinflamm. 2005, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Lin, J.; Ringman, J.; Kermani-Arab, V.; Tsao, G.; Patel, A.; Lossinsky, A.S.; Graves, M.C.; Gustavson, A.; Sayre, J. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2005, 3, 221–232. [Google Scholar] [CrossRef]
- Simard, A.R.; Soulet, D.; Gowing, G.; Julien, J.P.; Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 2006, 4, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, L.; Sun, X.H. Monocytes and Alzheimer’s disease. Neurosci. Bull. 2011, 2, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Zhang, L.; Gan, X.; Sherry, B.; Taub, D.; Graves, M.C.; Hama, S.; Way, D.; Weinand, M.; Witte, M. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood-brain barrier model. Mol. Med. 1998, 4, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Calabrese, E.; Marventano, I.; Piancone, F.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav. Immun. 2011, 25, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Calabrese, E.; Piancone, F.; Rainone, V.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer Rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Nielsen, J.L.; Dueholm, M.S.; Wetzel, R.; Otzen, D.; Nielsen, P.H. Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol. 2007, 9, 3077–3090. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Nielsen, J.L.; Otzen, D.; Nielsen, P.H. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl. Environ. Microbiol. 2008, 74, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Jordal, P.B.; Dueholm, M.S.; Larsen, P.; Petersen, S.V.; Enghild, J.J.; Christiansen, G.; Højrup, P.; Nielsen, P.H.; Otzen, D.E. Widespread Abundance of Functional Bacterial Amyloid in Mycolata and Other Gram-Positive Bacteria. Appl. Environ. Microbiol. 2009, 75, 4101–4110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, K.; Boles, B.R. Microbial amyloids–functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tükel, C.; Nishimori, J.H.; Wilson, R.P.; Winter, M.G.; Keestra, A.M.; van Putten, J.P.; Bäumler, A.J. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid fromenterobacterial biofilms. Cell. Microbiol. 2010, 12, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; La Rosa, F.; Piancone, F.; Zoppis, M.; Marventano, I.; Calabrese, E.; Rainone, V.; Nemni, R.; Mancuso, R.; Clerici, M. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C. Systemic inflammation and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2013, 39, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Kubilus, J.K.; Lee, J.; Ryu, H.; Beesen, A.; Zucker, B.; Smith, K.; Kowal, N.W.; Ratan, R.R.; Luthi-Carter, R. Histone Deacetylase Inhibition by Sodium Butyrate Chemotherapy Ameliorates the Neurodegenerative Phenotype in Huntington’s Disease Mice. J. Neurosci. 2003, 23, 9418–9427. [Google Scholar] [CrossRef] [PubMed]
- Gardian, G.; Browne, S.E.; Choi, D.K.; Klivenyi, P.; Gregorio, J.; Kubilus, J.K.; Ryu, H.; Langley, B.; Ratan, R.R.; Ferrante, R.J. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington’s disease. J. Biol. Chem. 2005, 280, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut micobiota influence Blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Saresella, M.; Marventano, I.; Piancone, F.; Zoia, C.P.; Conti, E.; Ripamonti, E.; Ferrante, C.; and Clerici, M. Stavudine Reduces NLRP3-Inflammasome Activation and Upregulates Amyloid-beta Autophagy. bioRxiv 2018. [Google Scholar] [CrossRef]
- du Bois, T.M.; Deng, C.; Bell, W.; Huang, X.F. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience 2006, 139, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Khazanehei, H.; Jones, P.J.; Khafipour, E. Interactions between Obesity Status and Dietary Intake of Monounsaturated and Polyunsaturated Oils on Human Gut Microbiome Profiles in the Canola Oil Multicenter Intervention Trial (COMIT). Front. Microbiol. 2016, 7, 1612. [Google Scholar] [CrossRef] [PubMed]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J.H. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfego, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martinez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Byerley, L.O.; Samuelson, D.; Blanchard, E.; Luo, M.; Lorenzen, B.N.; Banks, S.; Ponder, M.A.; Welsh, D.A.; Taylor, C.M. Changes in the Gut Microbial Communities Following Addition of Walnuts to the Diet. J. Nutr. Biochem. 2017, 48, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Basun, H.; Cederholm, T.; Faxén-Irving, G.; Garlind, A.; Grut, M.; Vedin, I.; Palmblad, J.; Wahlund, L.O.; Eriksdotter-Jönhagen, M. Omega-3 supplementation in mild to moderate Alzheimer’s disease: Effects on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry. 2008, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Childs, C.E.; Calder, P.C.; Rogers, P.J. No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’s Disease: A Randomised Controlled Trial. Int. J. Mol. Sci. 2015, 16, 24600–24613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; Schultzberg, M.; Basun, H.; Cederholm, T. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients During Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimers Dis. 2015, 48, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Zandi, P.P.; Tucker, K.L.; Fitzpatrick, A.L.; Kuller, L.H.; Fried, L.P.; Burke, G.L.; Carlson, M.C. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005, 65, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Feskens, E.J.; Launer, L.J.; Kromhout, D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997, 145, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Famenini, S.; Rigali, E.A.; Olivera-Perez, H.M.; Dang, J.; Chang, M.T.; Halder, R.; Rao, R.V.; Pellegrini, M.; Porter, V.; Bredesen, D. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation. FASEB J. 2017, 31, 148–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukiw, W.J.; Cui, J.G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; Rossi, P. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid. Based Complement. Alternat. Med. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Cesaroni, V.; Brandalise, F.; Occhinegro, A.; Ratto, D.; Perrucci, F.; Lanaia, V.; Girometta, C.; Orrù, G.; Savino, E. Effects of Hericium erinaceus supplementation in wild-type mice support a dual-process model of recognition memory. Int. J. Med. Mushrooms 2018, 20, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code 634 of acute inflammation: Novel pro-resolving lipid mediators 635 in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; O’Reilly, J.-A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS ONE 2014, 9, e106503. [Google Scholar] [CrossRef]
- Kobayashi, Y.H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]

| Author | Studied Population | Study Design | Supplement | Supplementation Duration | Methods | Results |
|---|---|---|---|---|---|---|
| Watson et at., 2017, [73] | 20 middle-aged healthy people | RCT | 4 g of mixed DHA/EPA as capsules and drink | 8 weeks | NGS of 16S rRNA gene, V4 region | At phylum level: no difference for Firmicutes/Bacteroidetes ratio. At family level: increase in Clostridiaceae, Sutterellaceae and Akkermansiaceae. At genus level: increased abundance of Bifidobacterium Oscillospira and reduction of Caprococcus and Faecalibacterium genera. In drink group increased abundance of Lachnospira and Roseburia genera. |
| Pu et al., 2016, [74] | 25 people with risk of metabolic syndrome | RCT | 60 g of different unsaturated oil blend | 30 days | Pyrosequncing of 16S rRNA gene, V1-V3 regions | At phylum level: no difference. Reversible increase in Bifidobacterium, Lachnospira, Roseburia, and Lactobacillus |
| Noriega et al., 2016, [75] | Case report | one healthy 45-year-old man | 600 mg PUFA by fish protein diet | 2 weeks | NGS of 16S rRNA gene, V4 region | At phylum level: increase in Firmicutes and decrease in Bacteroidetes and Actinobacteria. At genus level: increase in Blautia, Roseburia, Coprococcus, Ruminococcus, and Subdoligranulum. Decrease in Faecalibacterium |
| Menni et al., 2017, [76] | 876 middle-aged and elderly women | 350 mg/day of DHA | NGS of 16S rRNA gene, V4 region | At phylum level: increase in Lachnospiraceae. | ||
| Ballego et al., 2016, [77] | 32 patients with type 2 diabetes | 100 g of sardines (about 3 g di DHA + EPA/day) | 5 day a week for 6 months | qPCR | At phylum level: Firmicutes/Bacteroidetes ratio decrease. At genus level: Prevotella increase |
| Author | Number of Patients (Dropout Excluded) | Age of Patients | Type of Patients | Study Design | Supplement | Supplementation Duration (months) | Methods | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Freund-Levi et al., 2006, [18] | 174 | 74 ± 9 | Mild to moderate AD | RCT | 1.7 g DHA + 0.6 g EPA | 6–12 | MMSE, ADAS-cog CDR | Did not delay the cognitive decline except in a subgroup with very mild AD |
| Freund-Levi et al., 2008, [82] | 174 | 74 ± 9 | Mild to moderate AD | RCT | 1.7 g DHA + 0.6 g EPA | 6–12 | NPI, MADRS, DAD, CGP | Does not ameliorate neuropsychiatric symptoms |
| Quinn et al., 2010, [83] | 402 | 76 ± 8.7 | Mild to moderate AD | RCT | 2 g DHA | 18 | MMSE ADAS-cog CDR ADCS-ADL NPI MRI | Does not slow the cognitive and functional decline in mild to moderate AD |
| Shinto et al., 2014, [84] | 34 | >55 | probable AD | RCT | 675 mg DHA + 975 mg EPA | 12 | MMSE ADAS-cog ADCS-ADL | Decrease the rate of decline in MMSE |
| Phillips et al., 2015, [85] | 76 | 71 ± 4.8 | 57 with cognitive impairment and 19 with AD | RCT | 600 mg DHA + 625 mg EPA | 4 | MMSE | Negligible benefits on mood and cognition in AD |
| Eriksdotter et al., 2015, [86] | 174 | 74 ± 9 | Mild to moderate AD | RCT | 1.7 g DHA + 0.6 g EPA | 6 | MMSE ADAS-cog | Stabilizes the cognitive performance of AD subjects. |
| Methods | Description |
|---|---|
| MMSE Mini-Mental State Examination | Evaluates memory, orientation, language, calculation, attention and visual construction. It is used in clinical practice. Scores range between 0 and 30. Typically, an A cut-off score of 23 or 24 has been used to define significant cognitive impairment. |
| ADAS-cog Alzheimer’s Disease Assessment Scale—Cognitive section | It is a reliable and sensitive psychometric method for the assessment of cognitive function in dementia. It is used to evaluate changes over time. It consists of 11 items and a score scale between 0 (no impairment) and 70 (very severe impairment). |
| ADCS-ADL Alzheimer Disease Cooperative Study—Activities of Daily Living | It measures the functional ability to assess basic living skills of daily life such as bathing, eating. |
| ADCS-IADL Alzheimer Disease Cooperative Study—Instrumental Activities of Daily Living | It measures the functional ability to assess complex skills of daily life such as preparing a meal, using the telephone, shopping. |
| CDR Clinical Dementia Rating Scale | It is based on caregiver interview. It assesses memory, judgment, orientation. Dementia is classified into questionable, mild, moderate, and severe. |
| NPI Neuropsychiatric Inventory | It assesses dementia-related behavioural symptoms. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Iorio, C.D.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. https://doi.org/10.3390/nu10091267
La Rosa F, Clerici M, Ratto D, Occhinegro A, Licito A, Romeo M, Iorio CD, Rossi P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients. 2018; 10(9):1267. https://doi.org/10.3390/nu10091267
Chicago/Turabian StyleLa Rosa, Francesca, Mario Clerici, Daniela Ratto, Alessandra Occhinegro, Anna Licito, Marcello Romeo, Carmine Di Iorio, and Paola Rossi. 2018. "The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials" Nutrients 10, no. 9: 1267. https://doi.org/10.3390/nu10091267





