Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A Randomized Controlled Trial
Abstract
:1. Introduction
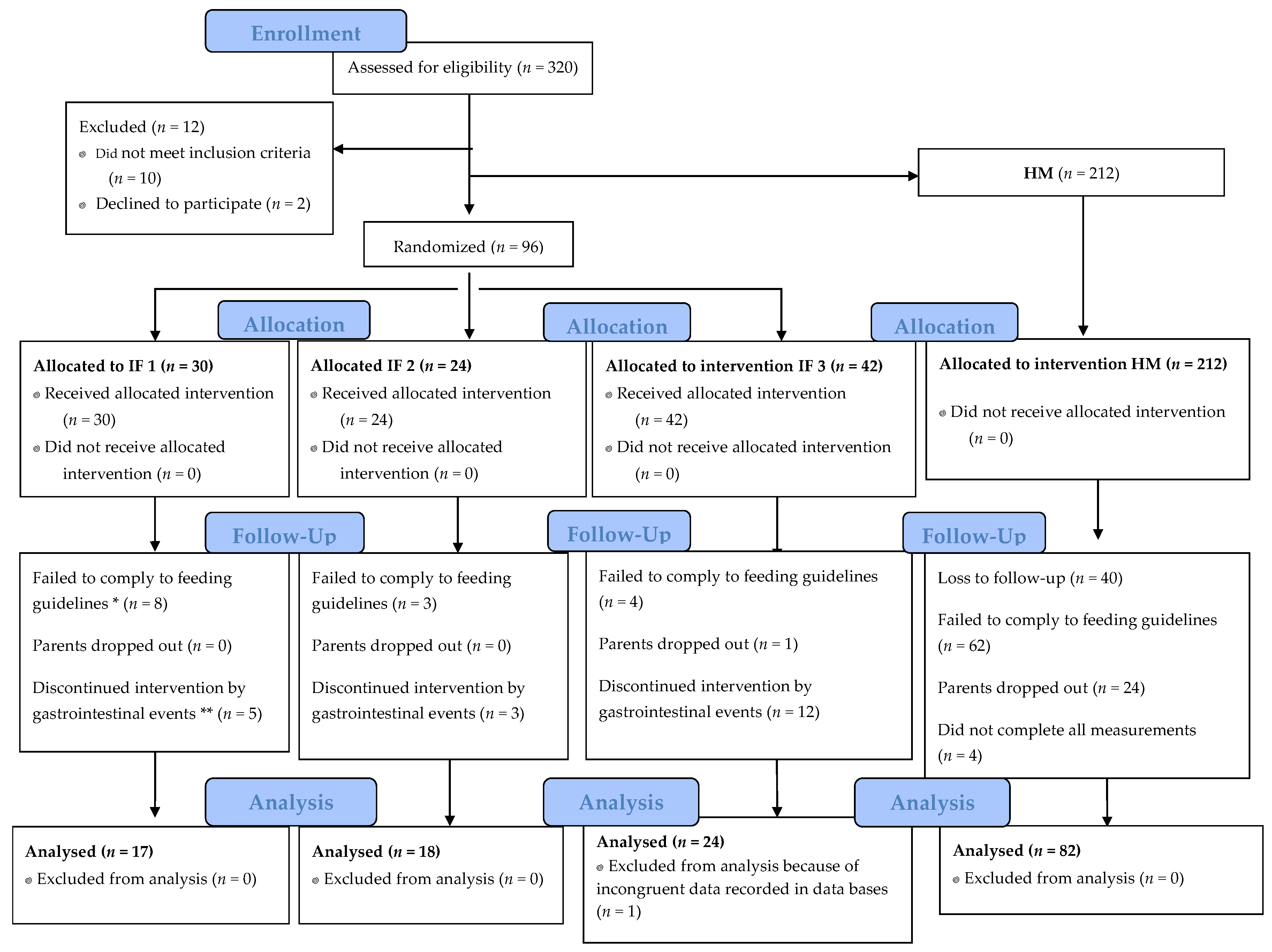
2. Subjects and Methods
2.1. Study Design
2.2. Study Size and Power Calculation
2.3. Study Population and Ethics
2.4. Nutritional Composition of Study Formulas
2.5. Study Visits and Anthropometric Measures
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Subjects Characteristics
3.2. Growth Velocity
3.3. Anthropometric Measurements and Growth (Z-scores)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.; Fall, C. Infant nutrition and later health: A review of current evidence. Nutrients 2012, 4, 859–874. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Optimal Duration of Exclusive Breast-Feeding; Report of an Expert Consultation; WHO: Geneva, Switzerland, 2001; Available online: http://www.who.int/nutrition/publications/infantfeeding/WHO_NHD_01.09/en/ (accessed on 12 July 2017).
- World Health Organization (WHO). Babies and Mothers Worldwide Failed by Lack of Investment in Breastfeeding. Available online: http://www.who.int/mediacentre/news/releases/2017/lack-investment-breastfeeding/es/ (accessed on 10 October 2017).
- Committee on Nutrition; American Academy of Pediatrics. Formula feeding of term infants. In Pediatric Nutrition Handbook, 6th ed.; Kleinman, R.E., Ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2009; pp. 61–78. [Google Scholar]
- Cruz-Ángeles, L.J.L. Formulación de fórmulas infantiles. In Fórmulas Infantiles, Nutrición y Tecnología, 1st ed.; Rosado, J.L., Ed.; MGS Ediciones: Querétaro, Mexico, 2017; pp. 23–35. [Google Scholar]
- Lönnerdal, B.; Lien, E.L. Nutritional and physiologic significance of alpha-lactalbumin in infants. Nutr. Rev. 2003, 61, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Gao, M.; Holley, T.; Zhou, T.; Burgher, A.; Trabulsi, J.; Pramuk, K.; Nazzario, J. Amino acid composition and protein content of mature human milk from nine countries. FASEB J. 2009, 23, LB448. [Google Scholar]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Delegated Regulation (EU) 2016/127; Official Journal of the European Union: Brussels, Belgium, 2016; Available online: https://ec.europa.eu/food/safety/labelling_nutrition/special_groups_food/children (accessed on 8 July 2018).
- Lien, E.L. Infant formulas with increased concentrations of alpha-lactalbumin. Am. J. Clin. Nutr. 2003, 77, 1555S–1558S. [Google Scholar] [CrossRef] [PubMed]
- Heine, W.E.; Klein, P.D.; Reeds, P.J. The importance of α-lactalbumin in infant nutrition. J. Nutr. 1991, 121, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [PubMed]
- Fleddermann, M.; Demmelmair, H.; Grote, V.; Nikolic, T.; Trisic, B.; Koletzko, B. Infant formula composition affects energetic efficiency for growth: The BeMIM study, a randomized controlled trial. Clin. Nutr. 2014, 33, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Subias, J.E.; Scaglioni, S.; Verduci, E.; Dain, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar] [PubMed]
- Kwon, G.; Marshall, C.A.; Pappan, K.L.; Remedi, M.S.; McDaniel, M.L. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes 2004, 53, S225–S232. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Excessive leucine-mTORC1-signalling of cow milk-based infant formula: The missing link to understand early childhood obesity. J. Obes. 2012, 2012, 197653. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Elvira Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Stettler, N.; Kumanyika, S.K.; Katz, S.H.; Zemel, B.S.; Stallings, V.A. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am. J. Clin. Nutr. 2003, 77, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Karaolis-Danckert, N.; Gunther, A.L.B.; Kroke, A.; Hornberg, C.; Buyken, A.E. How early dietary factors modify the effect of rapid weight gain in infancy on subsequent body-composition development in term children whose birth weight was appropriate for gestational age. Am. J. Clin. Nutr. 2007, 86, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Leunissen, R.W.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. J. Am. Med. Assoc. 2009, 301, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Janszen, D.B.; Lonnerdal, B.; Lien, E.L.; Pramuk, K.P.; Kuhlman, C.F. A multinational study of alpha-lactalbumin concentrations in human milk. J. Nutr. Biochem. 2004, 15, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 3, 712S–717S. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Vázquez, M.A. Análisis comparativo de las fórmulas infantiles comercializadas en México. In Fórmulas Infantiles, Nutrición y Tecnología, 1st ed.; Rosado, J.L., Ed.; MGS Ediciones: Querétaro, Mexico, 2017; pp. 303–304. [Google Scholar]
- Trabulsi, J.; Capeding, R.; Lebumfacil, J.; Ramanujam, K.; Feng, P.; McSweeney, S.; Harris, B.; DeRusso, P. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur. J. Clin. Nutr. 2011, 65, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Yan, J.; Bylsma, L.C.; Northington, R.S.; Grathwohl, D.; Steenhout, P.; Erdmann, P.; Spivey-Krobath, E.; Haschke, F. Growth of infants consuming whey-predominant term infant formulas with a protein content of 1.8 g/100 kcal: A multicenter pooled analysis of individual participant data. Am. J. Clin. Nutr. 2016, 104, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Inostroza, J.; Haschke, F.; Steenhout, P.; Grathwohl, D.; Nelson, S.E.; Ziegler, E.E. Low-Protein formula slows weight gain in infants of overweight mothers: A rondomized trial. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dallal, G. Webpage: randomization.com. Available online: http://www.randomization.com (accessed on 5 February 2016).
- Codex Alimentarius Commission. Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants; CODEX STAN 72-1981; Codex Alimentarius Commission: Rome, Italy, 1981. [Google Scholar]
- Duarte-Vázquez, M.Á.; García-Ugalde, C.; Villegas-Gutiérrez, L.M.; García-Almendárez, B.E.; Rosado, J.L. Production of Cow ’s Milk Free from Beta-Casein A1 and Its Application in the Manufacturing of Specialized Foods for Early Infant Nutrition. Foods 2017, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.E. Estudio del Perfil Proteínico de las Fórmulas Infantiles de Inicio Comercializadas en México. Master’s Thesis, Universidad Autónoma de Querétaro, Querétaro, Mexico, December 2014. [Google Scholar]
- Ballard, O.M.A. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- García, O.P.; Ronquillo, D.; Caamaño, M.C.; Martínez, G.; Camacho, M.; López, V.; Rosado, J.L. Zinc, iron and vitamins A, C and e are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients 2013, 5, 5012–5030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Anthro (version 3.2.2, January 2011) and Macros. Available online: https://www.who.int/childgrowth/software/en/ (accessed on 30 March 2016).
- Räihä, N.C.R.; Fazzolari-Nesci, A.; Cajozzo, C.; Puccio, G.; Monestier, A.; Moro, G.; Minoli, I.; Haschke-Becher, E.; Bachmann, C.; Van’t Hof, M.; et al. Whey Predominant, Whey Modified Infant Formula with Protein/energy Ratio of 1.8 g/100 kcal: Adequate and Safe for Term Infants From Birth to Four Months. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Liotto, N.; Orsi, A.; Menis, C.; Piemontese, P.; Morlacchi, L.; Condello, C.; Giannì, M. Clinical evaluation of two different protein content formulas fed to full-term healthy infants: A randomized controlled trial. Pediatrics 2018, 18, 56. [Google Scholar]
- Ong, K.K.; Loos, R.J. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006, 95, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Mori, T.; Huang, R.-C.; Marsh, J.; Pennell, C.E.; Chivers, P.T.; Hands, B.P.; Jacoby, P.; Rzehak, P.; Koletzko, B.V.; et al. Early infant feeding and adiposity risk: From infancy to adulthood. Ann. Nutr. Metab. 2014, 64, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Redsell, S.A.; Swift, J.A.; Yang, M.; Glazebrook, C.P. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch. Dis. Child. 2012, 97, 1019–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.M.; Harris, B.J.; Lien, E.L.; Pramuk, K.; Trabulsi, J. Alpha-lactalbumin-rich infant formula fed to healthy term infants in a Lower multicenter study: Plasma essential amino acids and gastrointestinal tolerance. Eur. J. Clin. Nutr. 2008, 62, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, I.E.; Ivarsson, S.A.; Räihä, N.C. Protein intake in early infancy: Effects on plasma amino acid concentrations, insulin metabolism, and growth. Pediatr. Res. 1989, 26, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Holly, J.M.P.; Smith, G.D.; Ness, A.R.; Emmett, P.; Rogers, I.; Gunnell, D.; Team, A.S. Could associations between breastfeeding and insulin-like growth factors underlie associations of breastfeeding with adult chronic disease? The Avon Longitudinal Study of Parents and Children. Clin. Endocrinol. 2005, 62, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B. The potential mechanistic link between allergy and obesity development and infant formula feeding. Asthma Clin. Immunol. Rev. 2014, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano, J.; Luque, V.; Ferre, N.; Zaragoza-Jordana, M.; Grote, V.; Koletzko, B.; Gruszfeld, D.; Socha, P.; Dain, E.; Van Hees, J.N.; et al. Increased protein intake augments kidney volume and function in healthy infants. Int. Soc. Nephrol. 2011, 79, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative Effects of A1 versus A2 Beta-Casein on Gastrointestinal Measures: A Blinded Randomised Cross-over Pilot Study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.L.; Davis, A.M.; multi-center group. Growth and safety of a reduced protein formula enriched with bovine alpha-lactalbumin in term infants. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 170–176. [Google Scholar] [CrossRef] [PubMed]
| Infant Formulas | HM ** | |||
|---|---|---|---|---|
| IF 1 * | IF 2 * | IF 3 * | ||
| Energy (kcal/dL) | 65 | 68 | 70 | 63.6 ± 4.5 |
| Protein profile | ||||
| Whey:Casein ratio | 65:35 | 65:35 | 60:40 | 70:30 |
| Protein content (g/dL) a | 1.0 | 1.3 | 1.5 | 0.9 ± 0.2 |
| Protein (g/100 kcal) | 1.43 | 1.9 | 2.18 | 1.28 |
| Beta-casein variant b | BCA2 | BCA2 | BCA1/BCA2 | Human beta-casein |
| Alpha-lactalbumin (%) | 26 | 26 | 11 | 33 |
| IF 1 | IF 2 | IF 3 | HM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 17 | n = 18 | n = 24 | n = 82 | ||||||
| Exclusively IF | 3 | 17.6% | 5 | 27.8% | 6 | 25.0% | 1 | 1.2% | |
| Exclusively Breastfed | 6 | 35.3% | 2 | 11.1% | 7 | 29.2% | 67 | 81.7% | |
| Mixed feeding | 8 | 47.1% | 11 | 61.1% | 11 | 45.8% | 14 | 17.1% | |
| Data are shown as: n (%) | |||||||||
| Feeding Group | p | ||||
|---|---|---|---|---|---|
| IF 1 | IF 2 | IF 3 | HM | ||
| n = 17 | n = 18 | n = 24 | n = 82 | ||
| Infant Characteristics | |||||
| Age at baseline (d) | 19 ± 11 | 21 ± 12 | 22 ± 11 | 21 ± 12 | 0.803 |
| Weight at birth (g) | 3116 ± 250 | 2789± 391 a | 2999 ± 399 | 3131 ± 380 | 0.004 |
| Length at birth (cm) | 50.0 ± 1.6 | 49.1 ±2.7 | 49.2 ± 2.1 | 49.9 ± 2.1 | 0.349 |
| Gestational age (wk) | 39.1 ±0.9 | 38.3 ± 1.7 | 38.6 ± 1.3 | 39.0 ± 1.1 | 0.112 |
| Delivery | |||||
| Caesarean | 6 (35%) | 7 (39%) | 11 (46%) | 36 (45%) | 0.981 |
| Forceps | 1 (7%) | 0 (0) | 1 (4%) | 1 (1%) | |
| Normal vaginal | 10 (58%) | 12 (61%) | 12 (50%) | 45 (54%) | |
| Sex | |||||
| Male | 8 (47%) | 12 (61%) | 10 (42%) | 44 (54%) | 0.467 |
| Female | 9 (53%) | 7 (39%) | 14 (58%) | 38 (46%) | |
| Mother’s Characteristics | |||||
| Age at baseline (y) | 21.1 ± 4.2 | 26.6 ± 7.8 | 24.9 ± 5.2 | 25 ± 5.4 | 0.074 |
| Mother’s education (y) | 8.0 ± 2.6 | 7.7 ± 3.3 | 8.9 ± 3.0 | 8.9 ± 2.2 | 0.211 |
| Crowding (people per room) | 3.0 ± 1.6 | 2.5 ± 0.8 b | 3.0± 1.6 | 3.6 ± 1.8 | 0.041 |
| Growth Velocity | Feeding Group | ||||
|---|---|---|---|---|---|
| IF 1 | IF 2 | IF 3 | HM | p | |
| n = 17 95% CI | n = 18 95% CI | n = 24 95% CI | n = 82 95% CI | ||
| Weight gain (g/day) | 25.8 (21.9, 29.2) a | 32.3 (28.9, 35.8) | 31.5 (28.5, 34.5) | 27.0 (25.5, 28.7) b | 0.001 |
| Length gain (cm/mo) | 2.4 (2.1, 2.7) | 2.6 (2.3, 2.9) | 2.6 (2.3, 2.8) | 2.4 (2.3, 2.6) | 0.646 |
| Head circumference gain (cm/mo) | 1.2 (1.1, 1.4) | 1.3 (1.2, 1.5) | 1.3 (1.0, 1.4) | 1.2 (1.1, 1.3) | 0.530 |
| Feeding Group | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IF 1 | IF 2 | IF 3 | HM | ||||||
| n = 17 | 95% CI | n = 18 | 95% CI | n = 24 | 95% CI | n = 82 | 95% CI | ||
| Weight (g) | |||||||||
| Baseline | 3617.6 | (3333.7, 3877.4) | 3276.6 a | (3003.5, 3549.8) | 3679.1 | (3310.0, 3911.2) | 3718.7 | (3587.4, 3850.1) | 0.042 |
| 4 mos. of age * | 6330.7 | (6011.0, 6650.4) | 6482.8 | (6174.7, 6791.0) | 6674.1 | (6413.0, 6935.1) | 6470.4 | (6328.3, 6612.5) | 0.396 |
| Adjusted change | 2697.4 | (2738.8, 3015.1) | 2856.4 | (2549.0, 3163.8) | 2997.4 | (2737.5, 3257.2) | 2834.1 | (2692.9, 2975.4) | 0.531 |
| Length (cm) | |||||||||
| Baseline | 50.1 | (48.9, 51.2) | 48.8 | (47.7, 49.9) | 50.3 | (48.8, 51.3) | 50.5 | (50.0, 51.0) | 0.082 |
| 4 mos. of age * | 59.9 | (58.9, 60.7) | 59.9 | (59.0, 60.7) | 60.6 | (59.9, 61.3) | 60.4 | (60.0, 60.8) | 0.365 |
| Adjusted change | 9.7 | (8.8, 10.6) | 9.8 | (8.9, 10.6) | 10.4 | (9.6, 11.1) | 10.3 | (9.9, 10.7) | 0.472 |
| Head Circumference (cm) | |||||||||
| Baseline | 35.6 | (34.9, 36.4) | 34.5 b | (33.6, 35.3) | 35.7 | (34.9, 36.5) | 35.8 | (35.5, 36.2) | 0.018 |
| 4 mos. of age * | 40.2 | (39.7, 40.7) | 40.6 | (40.1, 41.1) | 40.9 | (40.4, 41.3) | 40.8 | (40.5, 41.0) | 0.180 |
| Adjusted change | 4.9 | (4.5, 5.4) | 4.8 | (4.4, 5.3) | 5.2 | (4.8, 5.6) | 5.2 | (4.9, 5.3) | 0.494 |
| Feeding Group | HM | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IF 1 | IF 2 | IF 3 | |||||||
| n = 17 | 95% CI | n = 18 | 95% CI | n = 24 | 95% CI | n = 82 | 95% CI | ||
| WAZ | |||||||||
| Baseline | −0.5 | (−1.0, −0.1) | −1.3 a | (−1.7, −0.9) | −0.9 | (−1.2, −0.5) | −0.5 | (−0.7, −0.3) | 0.003 |
| 1 mo. | −0.8 | (−0.9, −0.3) | −0.7 | (−0.8, −0.5) | −0.5 | (−0.7, −0.4) | −0.6 | (−0.7, −0.5) | 0.065 |
| 4 mos. | −0.6 | (−1.0, −0.3) | −0.1 b | (−0.4, 0.3) | 0.0 | (−0.4, 0.3) | −0.4 | (−0.5, −0.2) | 0.049 |
| WLZ | |||||||||
| Baseline | 0.7 | (0.18, 1.2) | 0.2 | (−0.3, 0.7) | 0.5 | (0.0, 0.9) | 0.6 | (0.4, 0.8) | 0.468 |
| 1 mo. | 1.0 | (0.6, 1.3) | 0.9 | (0.5, 1.2) | 0.9 | (0.6, 1.2) | 1.0 | (0.8, 1.1) | 0.931 |
| 4 mos. | 0.7 | (0.3, 1.1) | 1.1 | (0.8, 1.5) | 1.0 | (0.7, 1.3) | 0.7 | (0.5, 0.9) | 0.139 |
| LAZ | |||||||||
| Baseline | −1.3 | (−1.8, −0.8) | −2 a | (−1.8, −0.8) | −1.6 | (−2.1, −1.2) | −1.2 | (−1.4, −0.9) | 0.016 |
| 1 mo. | −1.6 | (−1.8, −1.4) | −1.5 | (−1.7, −1.3) | −1.4 | (−1.6, −1.4) | −1.5 | (−1.6, −1.4) | 0.361 |
| 4 mos. | −1.5 | (−1.9, −1.1) | −1.1 | (−1.5, −0.7) | −1 | (−1.4, −0.6) | −1.2 | (−1.4, −1.0) | 0.302 |
| HCAZ | |||||||||
| Baseline | −0.2 | (−0.7, 0.2) | −1.3 c | (−1.8, −0.8) | −0.5 | (−0.9, −0.1) | −0.2 | (−0.4, −0.0) | 0.001 |
| 1 mo. | −0.5 | (−0.7, −0.3) | −0.4 | (−0.6, −0.2) | −0.4 | (−0.5, −0.2) | −0.4 | (−0.5, −0.3) | 0.578 |
| 4 mos. | −0.5 | (−0.9, −0.2) | −0.2 | (−0.5, 0.1) | −0.1 | (−0.4, 0.1) | −0.3 | (−0.5, −0.2) | 0.184 |
| BMIZ | |||||||||
| Baseline | 0.2 | (−0.2, 0.7) | −0.3 | (−0.8, 0.0) | 0 | (−0.4, 0.4) | 0.2 | (0.0, 0.4) | 0.122 |
| 1 mo. | 0.1 | (−0.1, 0.4) | −0.2 | (−0.1, 0.3) | 0.3 | (0.1, 0.5) | 0.3 | (0.2, 0.4) | 0.337 |
| 4 mos. | 0.3 | (−0.1, 0.7) | 0.8 | (0.4, 1.2) | 0.7 | (0.4, 1.1) | 0.4 | (0.3, 0.6) | 0.107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oropeza-Ceja, L.G.; Rosado, J.L.; Ronquillo, D.; García, O.P.; Caamaño, M.D.C.; García-Ugalde, C.; Viveros-Contreras, R.; Duarte-Vázquez, M.Á. Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A Randomized Controlled Trial. Nutrients 2018, 10, 886. https://doi.org/10.3390/nu10070886
Oropeza-Ceja LG, Rosado JL, Ronquillo D, García OP, Caamaño MDC, García-Ugalde C, Viveros-Contreras R, Duarte-Vázquez MÁ. Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A Randomized Controlled Trial. Nutrients. 2018; 10(7):886. https://doi.org/10.3390/nu10070886
Chicago/Turabian StyleOropeza-Ceja, Lorena G., Jorge L. Rosado, Dolores Ronquillo, Olga P. García, María Del C. Caamaño, Carlos García-Ugalde, Rubí Viveros-Contreras, and Miguel Ángel Duarte-Vázquez. 2018. "Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A Randomized Controlled Trial" Nutrients 10, no. 7: 886. https://doi.org/10.3390/nu10070886




