Mangosteen Extract Shows a Potent Insulin Sensitizing Effect in Obese Female Patients: A Prospective Randomized Controlled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Protocol
2.3. Lifestyle Intervention
2.4. Outcome Measures
2.4.1. Anthropometric Measurements and Vital Parameters
2.4.2. Laboratory Assessments
2.4.3. Body Composition
2.5. Product Description
2.6. Statistical Analysis
3. Results
3.1. Population
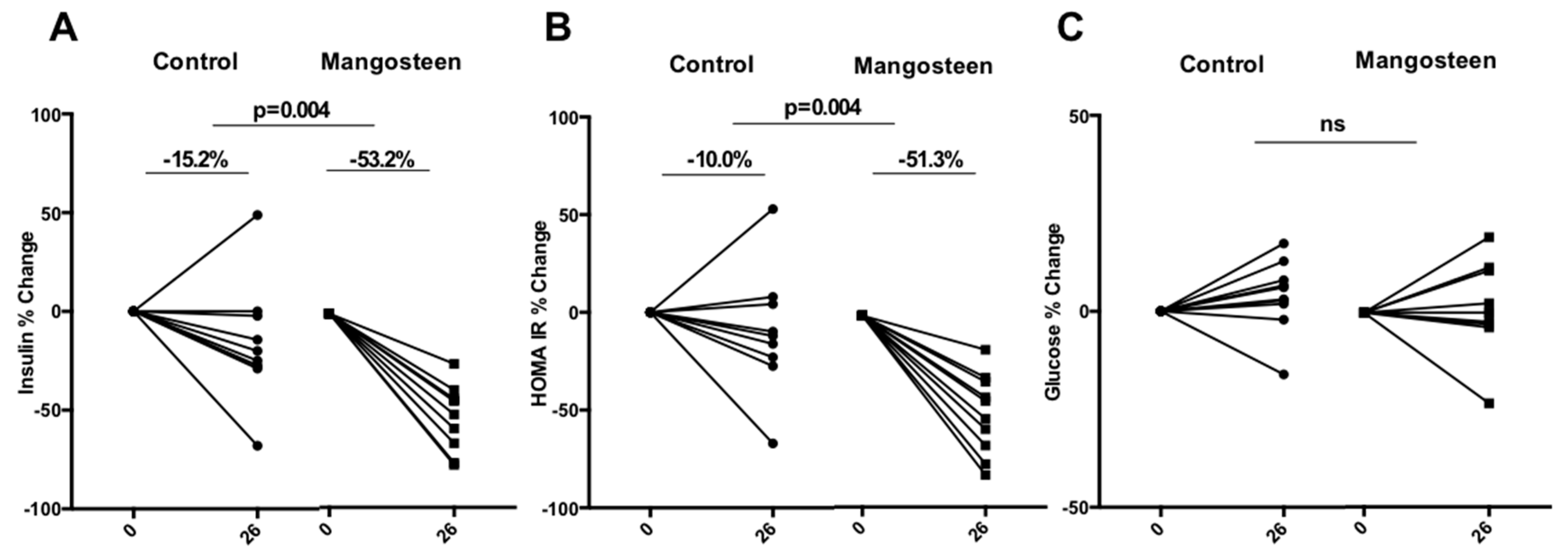
3.2. Glucose Metabolism
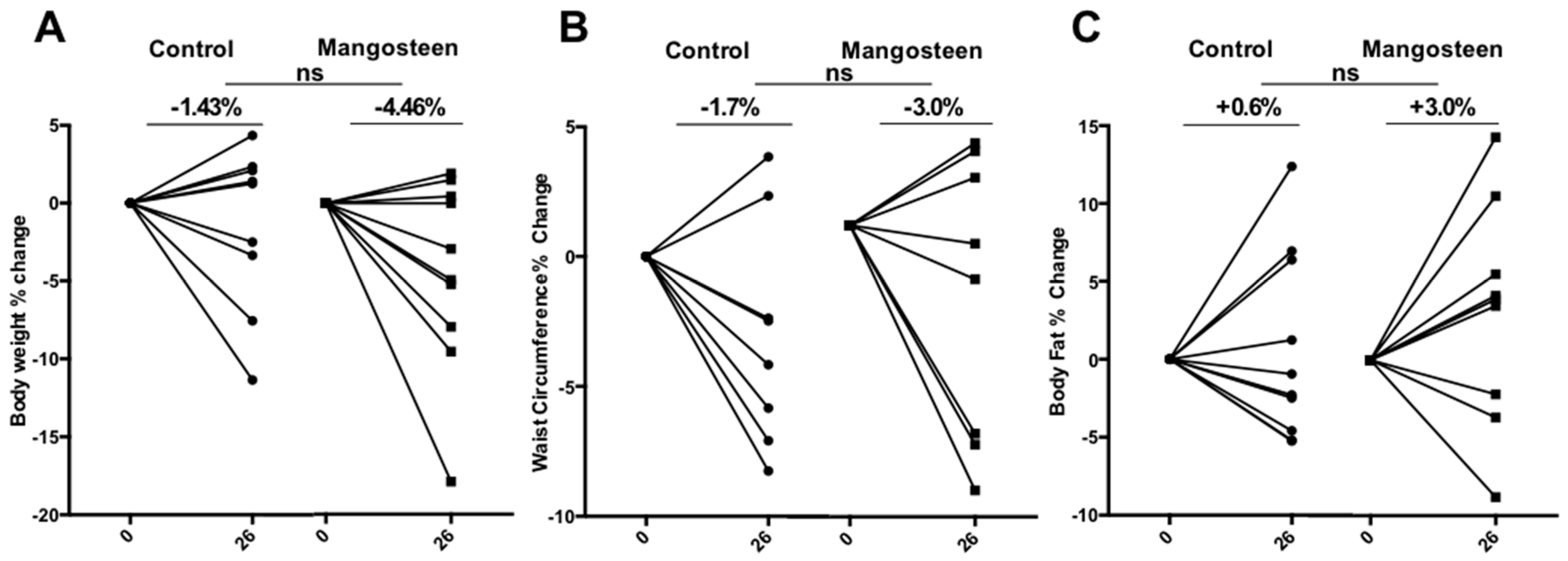
3.3. Anthropometric Parameters
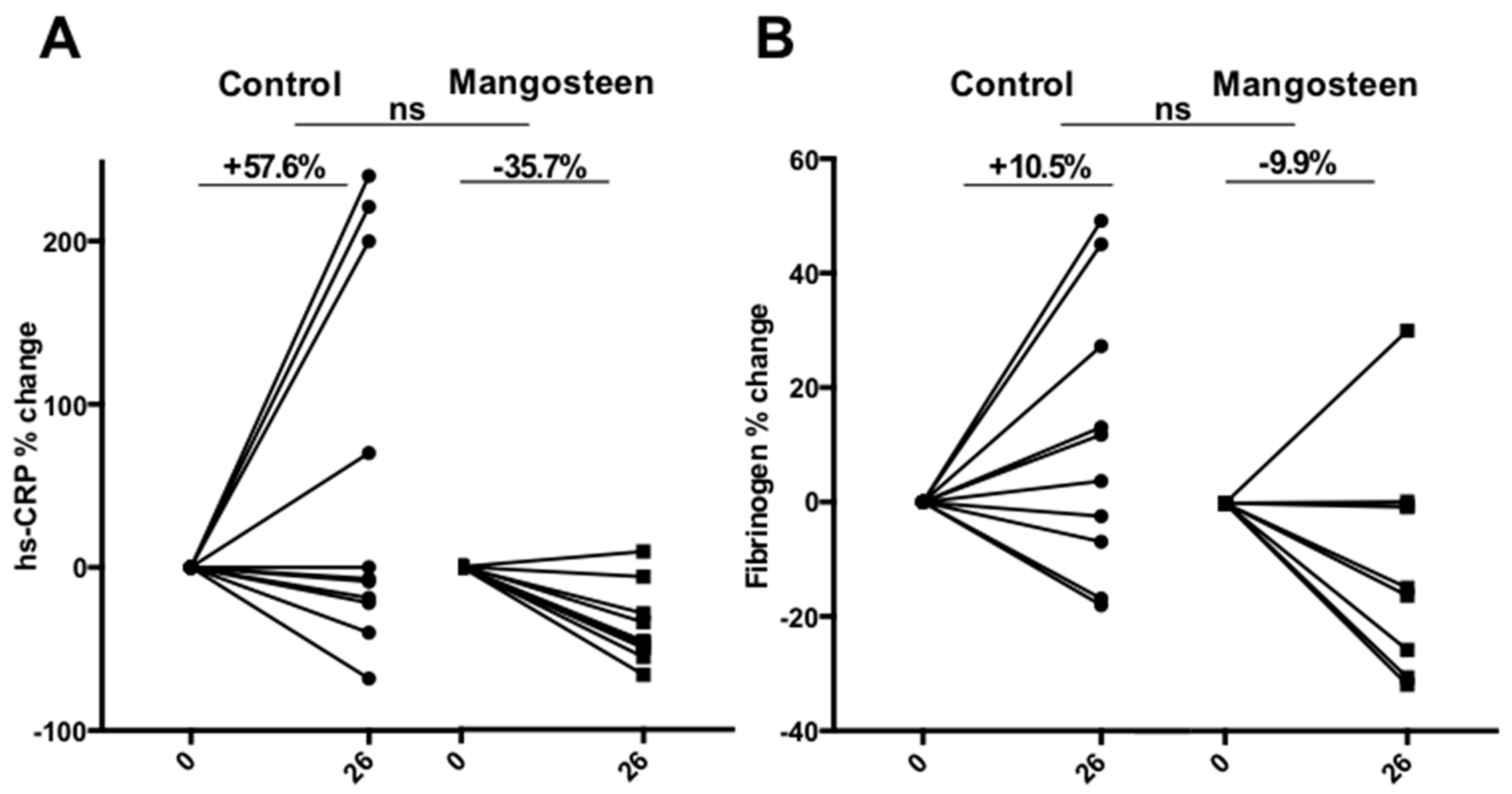
3.4. Inflammation Markers
3.5. Lipids Profile
3.6. Safety and Tolerability
3.7. Compliance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight Factsheet. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 8 April 2017).
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study. Diabetes Care 2012, 35, 731–737. [Google Scholar]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Romeo, G.R.; Lee, J.; Shoelson, S.E. Metabolic syndrome, insulin resistance, and roles of inflammation—Mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Suttirak, W.; Manurakchinakorn, S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014, 51, 3546–3558. [Google Scholar] [CrossRef] [PubMed]
- Jariyapongskul, A.; Areebambud, C.; Suksamrarn, S.; Mekseepralard, C. Alpha-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. Biomed. Res. Int. 2015, 2015, 785826. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. Alpha-glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from garcinia mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Tg Zakaria, T.M.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic activity of ethanolic extract of garcinia mangostana linn. In normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-S.; Kim, E.Y.; Han, L.; Kim, N.R.; Chin, Y.W. Xanthones with pancreatic lipase inhibitory activity from the pericarps of Garcinia mangostana L. Eur. J. Lipid Sci. Technol. 2016, 118, 1416–1421. [Google Scholar] [CrossRef]
- Quan, X.; Wang, Y.; Ma, X.; Liang, Y.; Tian, W.; Ma, Q.; Jiang, H.; Zhao, Y. Alpha-mangostin induces apoptosis and suppresses differentiation of 3t3-l1 cells via inhibiting fatty acid synthase. PLoS ONE 2012, 7, e33376. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Ryu, H.W.; So, Y.; Lee, C.W.; Jin, C.H.; Yook, H.S.; Jeong, Y.W.; Park, J.C.; Jeong, I.Y. Anti-inflammatory effect of mangostenone F in lipopolysaccharide-stimulated RAW264.7 macrophages by suppressing NF-Kappab and mapk activation. Biomol. Ther. 2014, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Wattanapiromsakul, C.; Mahabusarakam, W. Effects of compounds from garcinia mangostana on inflammatory mediators in raw264.7 macrophage cells. J. Ethnopharmacol. 2009, 121, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Bae, J.K.; Chae, H.S.; Kim, Y.M.; Sreymom, Y.; Han, L.; Jang, H.Y.; Chin, Y.W. Alpha-mangostin regulates hepatic steatosis and obesity through sirt1-ampk and ppargamma pathways in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 8399–8406. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Kim, Y.M.; Bae, J.K.; Sorchhann, S.; Yim, S.; Han, L.; Paik, J.H.; Choi, Y.H.; Chin, Y.W. Mangosteen extract attenuates the metabolic disorders of high-fat-fed mice by activating ampk. J. Med. Food 2016, 19, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.K.; Singh, B.B.; Barrett, M.L.; Singh, V.J. Evaluation of mangosteen juice blend on biomarkers of inflammation in obese subjects: A pilot, dose finding study. Nutr. J. 2009, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.S.; Peerson, J.; Mishra, A.T.; Sadasiva Rao, M.V.; Rajeswari, K.P. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity 2013, 21, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Kudiganti, V.; Kodur, R.R.; Kodur, S.R.; Halemane, M.; Deep, D.K. Efficacy and tolerability of meratrim for weight management: A randomized, double-blind, placebo-controlled study in healthy overweight human subjects. Lipids Health Dis. 2016, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Jette, M.; Sidney, K.; Blumchen, G. Metabolic equivalents (mets) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef] [PubMed]
- Mekseepralard, C.; Areebambud, C.; Suksamrarn, S.; Jariyapongskul, A. Effects of long-term alpha-mangostin supplementation on hyperglycemia and insulin resistance in type 2 diabetic rats induced by high fat diet and low dose streptozotocin. J. Med. Assoc. Thail. 2015, 98 (Suppl. S10), S23–S30. [Google Scholar]
- Nakatani, K.; Nakahata, N.; Arakawa, T.; Yasuda, H.; Ohizumi, Y. Inhibition of cyclooxygenase and prostaglandin e2 synthesis by gamma-mangostin, a xanthone derivative in mangosteen, in c6 rat glioma cells. Biochem. Pharmacol. 2002, 63, 73–79. [Google Scholar] [CrossRef]
- Yamakuni, T.; Aoki, K.; Nakatani, K.; Kondo, N.; Oku, H.; Ishiguro, K.; Ohizumi, Y. Garcinone b reduces prostaglandin e2 release and nf-kappab-mediated transcription in c6 rat glioma cells. Neurosci. Lett. 2006, 394, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Robidoux, J. Dyslipidemia management update. Curr. Opin. Pharmacol. 2017, 33, 47–55. [Google Scholar] [CrossRef] [PubMed]
| Components | Quantity (per Capsule) | Function |
|---|---|---|
| Mangosteen fruit pulp extract | 400 mg titrated to 40% in α and γ mangostins (160 mg) | Active ingredient |
| Gelatin | 95 mg | External coating |
| Magnesium salts of fatty acids and silicon dioxide | 5 mg | Anti-caking |
| Control (n = 10) | Mangosteen (n = 10) | p | |
|---|---|---|---|
| Age (years) | 46.00 ± 12.009 | 43.70 ± 2.248 | 0.677 |
| BMI (kg/m2) | 37.60 ± 7.043 | 37.10 ± 4.725 | 0.854 |
| Body weight (kg) | 101.90 ± 23.662 | 101.10 ± 16.690 | 0.931 |
| Waist circumference (cm) | 120.40 ± 15.601 | 115.44 ± 8.748 | 0.413 |
| Body fat (%) | 40.20 ± 2.781 | 39.60 ± 3.777 | 0.691 |
| Serum glucose (mg/dL) | 93.20 ± 14.250 | 86.20 ± 8.979 | 0.205 |
| Serum insulin (mg/dL) | 19.11 ± 6.431 | 22.40 ± 15.072 | 0.553 |
| HOMA-IR | 4.44 ± 1.509 | 4.90 ± 3.872 | 0.745 |
| HbA1C (%) | 5.4 ± 0.31 | 5.4 ± 0.23 | 0.876 |
| Fibrinogen (mg/L) | 360.25 ± 65.876 | 454.78 ± 83.215 | 0.071 |
| hsCRP (mg/L) | 1.00 ± 1.155 | 0.80 ± 0.632 | 0.761 |
| Control (n = 10) | Mangosteen (n = 10) | C | M | C-M | |||
|---|---|---|---|---|---|---|---|
| Weeks | 0 | 26 | 0 | 26 | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p | p | p | |
| Total Cholesterol (mg/dL) | 203 ± 39 | 199 ± 46 | 193 ± 28 | 199 ± 34 | 0.682 | 0.391 | 0.815 |
| LDL-C (mg/dL) | 130 ± 40 | 128 ± 44 | 121 ± 21 | 123 ± 31 | 0.796 | 0.724 | 0.779 |
| HDL-C (mg/dL) | 49 ± 14 | 49 ± 10 | 50 ± 12 | 58 ± 13 | 0.876 | 0.024 | 0.528 |
| Triglycerides (mg/dL) | 124 ± 61 | 111 ± 41 | 92 ± 30 | 88 ± 21 | 0.222 | 0.530 | 0.322 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Gangitano, E.; Francomano, D.; Addessi, E.; Toscano, R.; Costantini, D.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; et al. Mangosteen Extract Shows a Potent Insulin Sensitizing Effect in Obese Female Patients: A Prospective Randomized Controlled Pilot Study. Nutrients 2018, 10, 586. https://doi.org/10.3390/nu10050586
Watanabe M, Gangitano E, Francomano D, Addessi E, Toscano R, Costantini D, Tuccinardi D, Mariani S, Basciani S, Spera G, et al. Mangosteen Extract Shows a Potent Insulin Sensitizing Effect in Obese Female Patients: A Prospective Randomized Controlled Pilot Study. Nutrients. 2018; 10(5):586. https://doi.org/10.3390/nu10050586
Chicago/Turabian StyleWatanabe, Mikiko, Elena Gangitano, Davide Francomano, Eliana Addessi, Raffaella Toscano, Daniela Costantini, Dario Tuccinardi, Stefania Mariani, Sabrina Basciani, Giovanni Spera, and et al. 2018. "Mangosteen Extract Shows a Potent Insulin Sensitizing Effect in Obese Female Patients: A Prospective Randomized Controlled Pilot Study" Nutrients 10, no. 5: 586. https://doi.org/10.3390/nu10050586






