Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Material and Methods
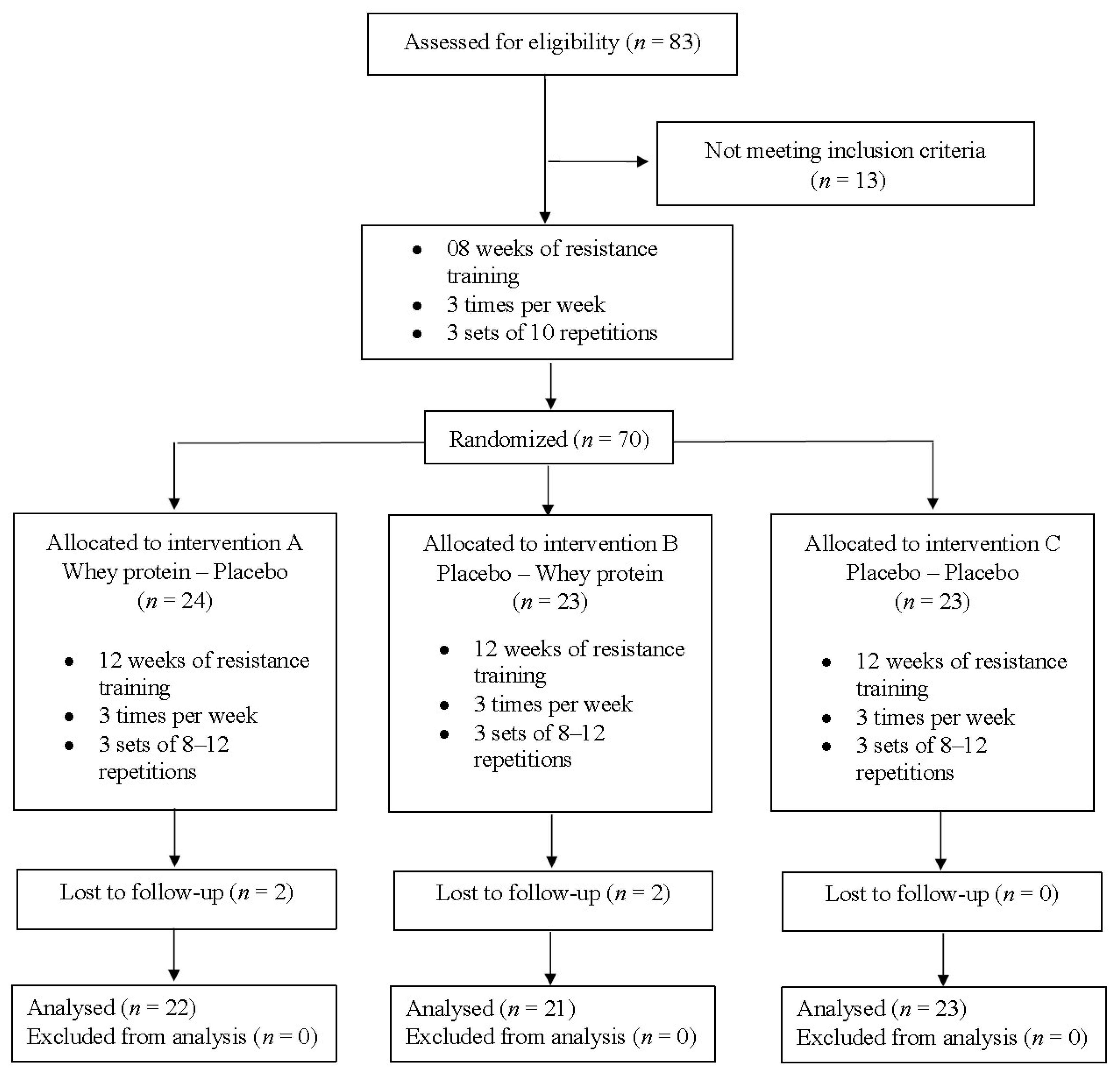
2.1. Experimental Design
2.2. Participants
2.3. Anthropometry
2.4. Body Composition
2.5. Muscular Strength
2.6. Functional Capacity
2.7. Dietary Intake
2.8. Supplementation Protocol
2.9. Resistance Training Program
3. Statistical Analyses
4. Results
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.; Nieman, D.C.; Swain, D.P. American College of Sports edicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. 1), A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.M.; van Loon, L.J.C. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv. Nutr. 2015, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Sugihara Junior, P.; Ribeiro, A.S.; Nabuco, H.C.G.; Fernandes, R.R.; Tomeleri, C.M.; Cunha, P.M.; Venturini, D.; Barbosa, D.S.; Schoenfeld, B.J.; Cyrino, E.S. Effects of Whey Protein Supplementation Associated with Resistance Training on Muscular Strength, Hypertrophy and Muscle Quality in Pre-Conditioned Older Women. Int. J. Sport Nutr. Exerc. Metab. 2017, 1–27. [Google Scholar] [CrossRef]
- Barbat-Artigas, S.; Pion, C.H.; Leduc-Gaudet, J.P.; Rolland, Y.; Aubertin-Leheudre, M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J. Am. Med. Dir. Assoc. 2014, 15, 303.e13–303.e20. [Google Scholar] [CrossRef] [PubMed]
- Colonetti, T.; Grande, A.J.; Milton, K.; Foster, C.; Alexandre, M.C.M.; Uggioni, M.L.R.; da Rosa, M.I. Effects of whey protein supplement in the elderly submitted to resistance training: Systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2017, 68, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, F.; Larumbe-Zabala, E. Effects of Whey Protein Alone or as Part of a Multi-ingredient Formulation on Strength, Fat-Free Mass, or Lean Body Mass in Resistance-Trained Individuals: A Meta-analysis. Sports Med. 2016, 46, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Contreras, B. The Muscle Pump: Potential Mechanisms and Applications for Enhancing Hypertrophic Adaptations. Strength Cond. J. 2013. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Tomeleri, C.M.; Souza, M.F.; Pina, L.C.; Schoenfeld, B.J.; Nascimento, M.A.; Venturini, D.; Barbosa, D.S.; Cyrino, E.S. Effect of resistance training on C-reactive protein, blood glucose and lipid profile in older women with differing levels of RT experience. AGE 2015, 37, 109. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Petrella, J.K.; Bamman, M.M. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl. Physiol. Nutr. Metab. 2009, 34, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient timing revisited: Is there a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahi, B.; Morais, J.A.; Gaudreau, P.; Payette, H.; Shatenstein, B. Energy and protein intakes and their association with a decline in functional capacity among diabetic older adults from the NuAge cohort. Eur. J. Nutr. 2016, 55, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kröger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkilä, A.T. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr. 2016, 115, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Chilibeck, P.D.; Facci, M.; Abeysekara, S.; Zello, G.A. Protein supplementation before and after resistance training in older men. Eur. J. Appl. Physiol. 2006, 97, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Jonkers, R.A.; Gleeson, B.G.; Beelen, M.; Meijer, K.; Savelberg, H.H.; Wodzig, W.K.; Dendale, P.; van Loon, L.J. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am. J. Clin. Nutr. 2009, 89, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Esmarck, B.; Andersen, J.L.; Olsen, S.; Richter, E.A.; Mizuno, M.; Kjaer, M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J. Physiol. 2001, 535, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Cribb, P.J.; Hayes, A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med. Sci. Sports Exerc. 2006, 38, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Tranchina, C.P.; Rashti, S.L.; Kang, J.; Faigenbaum, A.D. Effect of protein-supplement timing on strength, power, and body-composition changes in resistance-trained men. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.; Wilborn, C.; Urbina, S.L.; Hayward, S.E.; Krieger, J. Pre- versus post-exercise protein intake has similar effects on muscular adaptations. PeerJ 2017, 5, e2825. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Williams, A.G. The adaptations to strength training: Morphological and neurological contributions to increased strength. Sports Med. 2007, 37, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Tomeleri, C.M.; Ribeiro, A.S.; Souza, M.F.; Schiavoni, D.; Schoenfeld, B.J.; Venturini, D.; Barbosa, D.S.; Landucci, K.; Sardinha, L.B.; Cyrino, E.S. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp. Gerontol. 2016, 84, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Heshka, S.; Gallagher, D.; Kotler, D.P.; Mayer, L.; Albu, J.; Shen, W.; Freda, P.U.; Heymsfield, S.B. Intermuscular adipose tissue-free skeletal muscle mass: Estimation by dual-energy X-ray absorptiometry in adults. J. Appl. Physiol. 2004, 97, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Dantas, E.H.M.; Figueira, H.A.; Emygdio, R.F.; Vale, R.G.S. Functional Autonomy GDLAM Protocol Classification Pattern in Elderly Women. Ind. J. Appl. Res. 2014, 4, 262–266. [Google Scholar] [CrossRef]
- Monego, E.T.; Peixoto, M.D.R.G.; Santiago, R.D.A.C.; Gil, M.F.; Cordeiro, M.M.; Campos, M.I.; de Souza, R.G. Alimentos Brasileiros e Suas Porções: Um Guia para Avaliação do Consumo Alimentar; Rúbio: Rio de Janeiro, Brazil, 2013. (In Portuguese) [Google Scholar]
- Pinheiro, A.B.V.; Lacerda, E.M.D.A.; Benzecry, E.H.; Gomes, M.C.S.; da Costa, V.M. Tabela para Avaliação de Consumo Alimentar em Medidas Caseiras; Atheneu: Rio de Janeiro, Brazil, 2009. (In Portuguese) [Google Scholar]
- Ribeiro, A.S.; Deminice, R.; Schoenfeld, B.J.; Tomeleri, C.M.; Padilha, C.S.; Venturini, D.; Barbosa, D.S.; Sardinha, L.B.; Cyrino, E.S. Effect of Resistance Training Systems on Oxidative Stress in Older Women. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, E.; Bui, Q.-U.T.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Riek, S.; Carson, R.G. Neural adaptations to resistance training: Implications for movement control. Sports Med. 2001, 31, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Maior, A.S.; Alves, A. A contribuição dos fatores neurais em fases iniciais do treinamento de força muscular: Uma revisão bibliográfica. Motriz 2003, 9, 161–168. (In Portuguese) [Google Scholar]
- Phillips, S.M. Short-term training: When do repeated bouts of resistance exercise become training? Can. J. Appl. Physiol. 2000, 25, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.E.; Snijders, T.; Zulyniak, M.; Kumbhare, D.; Parise, G.; Chabowski, A.; Phillips, S.M. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLoS ONE 2017, 12, e0181387. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Soeters, P.B. The Biological Value of Protein. Nestle Nutr. Inst. Workshop Ser. 2015, 82, 39–51. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Marworth, J.F.; Figueiredo, V.C.; Della Gatta, P.A.; Petersen, A.C.; Mitchell, C.J.; Cameron-Smith, D. Dose-dependent increases in p70S6K phosphorylation and intramuscular branched-chain amino acids in older men following resistance exercise and protein intake. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Groen, B.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.; van Loon, L.J. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Saddler, N.I.; Devries, M.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: A parallel-group crossover study. Am. J. Clin. Nutr. 2016, 104, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Messier, V.; Suppere, C.; Briand, P.; Rabasa-Lhoret, R. Effect of cysteine-rich whey protein (immunocal(R)) supplementation in combination with resistance training on muscle strength and lean body mass in non-frail elderly subjects: A randomized, double-blind controlled study. J. Nutr. Health Aging 2015, 19, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Wu, Y.T.; Cheng, C.P.; Chen, H.C.; Huang, Y.C.; Chen, H.C.; Liou, T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Appendicular Lean Mass and Mortality among Prefrail and Frail Older Adults. J. Nutr. Health Aging 2017, 21, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Ansai, T.; Akifusa, S.; Soh, I.; Sonoki, K.; Takehara, T. High-level functional capacity and 4-year mortality in an 80-year-old population. Gerontology 2007, 53, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Ribeiro, A.S.; Schoenfeld, B.J.; Nascimento, M.A.; Tomeleri, C.M.; Souza, M.F.; Pina, F.L.; Cyrino, E.S. The improvement in walking speed induced by resistance training is associated with increased muscular strength but not skeletal muscle mass in older women. Eur. J. Sport Sci. 2017, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Dideriksen, K.J.; Reitelseder, S.; Petersen, S.G.; Hjort, M.; Helmark, I.C.; Kjaer, M.; Holm, L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand. J. Med. Sci. Sports 2011, 21, e372–e383. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, V.C.; Cameron-Smith, D. Is carbohydrate needed to further stimulate muscle protein synthesis/hypertrophy following resistance exercise? J. Int. Soc. Sports Nutr. 2013, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; McLaughlin, J. Carbohydrate ingestion during exercise: Effects on performance, training adaptations and trainability of the gut. Nestle Nutr. Inst. Workshop Ser. 2011, 69, 1–12. [Google Scholar] [PubMed]
- Trommelen, J.; van Loon, L.J. Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training. Nutrients 2016, 8, 763. [Google Scholar] [CrossRef] [PubMed]

| Pre | Post | Δ% | ES | p | |
|---|---|---|---|---|---|
| Protein (g/kg/day) | 0.92 ± 0.28 | 0.94 ± 0.28 | 2.2 | 0.07 | 0.499 |
| CHO (g/kg/day) | 3.07 ± 1.0 | 3.15 ± 1.0 | 2.5 | 0.08 | 0.324 |
| Lipids (g/kg/day) | 0.70 ± 0.2 | 0.73 ± 0.2 | 4.5 | 0.14 | 0.233 |
| Energy (kcal/kg/day) | 22.3 ± 6.5 | 23.0 ± 6.5 | 3.1 | 0.10 | 0.178 |
| SMM (kg) | 16.6 ± 2.6 | 17.1 ± 2.7 | 3.1 | 0.19 | <0.001 |
| ULLST (kg) | 3.8 ± 0.6 | 4.0 ± 0.6 | 3.5 | 0.21 | <0.001 |
| LLLST (kg) | 11.5 ± 1.6 | 11.8 ± 1.7 | 2.5 | 0.17 | <0.001 |
| Chest press (kg) | 40.6 ± 8.5 | 44.8 ± 8.7 | 10.3 | 0.48 | <0.001 |
| Knee extension (kg) | 46.0 ± 11.3 | 52.6 ± 11.7 | 14.4 | 0.57 | <0.001 |
| Preacher curl (kg) | 18.4 ± 3.8 | 21.8 ± 3.7 | 18.3 | 0.89 | <0.001 |
| Total strength (kg) | 105.0 ± 19.7 | 119.3 ± 20.3 | 13.6 | 0.71 | <0.001 |
| 10 MW (s) | 7.8 ± 1.1 | 7.4 ± 0.9 | −4.8 | 0.37 | <0.001 |
| RSP (s) | 12.4 ± 2.2 | 11.8 ± 1.7 | −5.2 | 0.33 | <0.001 |
| Whey Protein–Placebo (n = 22) | Placebo–Whey Protein (n = 21) | Placebo-Placebo (n = 23) | Interaction p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6-Week | 12-Week | 0 | 6-Week | 12-Week | 0 | 6-Week | 12-Week | ||
| Intake excluding whey/control | ||||||||||
| Protein (g/kg/day) | 0.92 ± 0.20 | 0.95 ± 0.36 | 0.96 ± 0.19 | 0.94 ± 0.36 | 0.98 ± 0.52 | 0.98 ± 0.24 | 0.95 ± 0.27 | 0.96 ± 0.26 | 0.99 ± 0.25 | 0.914 |
| CHO (g/kg/day) | 3.1 ± 0.96 | 3.1 ± 0.91 | 3.0 ± 0.77 | 3.2 ± 1.12 | 3.1 ± 1.24 | 3.1 ± 1.13 | 3.1 ± 0.94 | 3.1 ± 0.84 | 3.1 ± 0.72 | 0.968 |
| Lipids (g/kg/day) | 0.77 ± 0.29 | 0.72 ± 0.27 | 0.69 ± 0.19 | 0.76 ± 0.30 | 0.72 ± 0.21 | 0.74 ± 0.32 | 0.67 ± 0.17 | 0.73 ± 0.14 | 0.75 ± 0.34 | 0.485 |
| Energy (kcal/kg/day) | 22.9 ± 6.29 | 23.1 ± 7.76 | 23.2 ± 6.28 | 22.4 ± 7.75 | 23.3 ± 7.76 | 23.3 ± 6.28 | 22.4 ± 5.90 | 23.2 ± 4.6 | 23.4 ± 6.3 | 0.810 |
| Intake including whey/control | ||||||||||
| Protein (g/kg/day) | 0.92 ± 0.20 | 1.38 ± 0.42 *,§ | 1.38 ± 0.26 *,§ | 0.94 ± 0.36 | 1.42 ± 0.57 *,§ | 1.49 ± 0.46 *,§ | 0.95 ± 0.27 | 0.98 ± 0.30 | 1.0 ± 0.25 | <0.001 |
| CHO (g/kg/day) | 3.1 ± 0.96 | 3.7 ± 0.93 * | 3.6 ± 0.85 *,§ | 3.2 ± 1.12 | 3.4 ± 1.5 § | 3.6 ± 1.2 *,§ | 3.1 ± 0.94 | 4.2 ± 1.0 * | 4.2 ± 0.90 * | <0.001 |
| Lipids (g/kg/day) | 0.77 ± 0.29 | 0.72 ± 0.27 | 0.70 ± 0.17 | 0.76 ± 0.30 | 0.70 ± 0.29 | 0.74 ± 0.31 | 0.67 ± 0.17 | 0.72 ± 0.14 | 0.75 ± 0.33 | 0.279 |
| Energy (kcal/kg/day) | 22.9 ± 6.29 | 26.6 ± 7.0 | 26.1 ± 5.6 * | 22.4 ± 7.75 | 25.3 ± 10.4 | 28.0 ± 6.7 * | 22.4 ± 5.90 | 27.2 ± 4.6 * | 27.8 ± 6.8 * | 0.435 |
| Whey Protein–Placebo (n = 22) | Placebo–Whey Protein (n = 21) | Placebo-Placebo (n = 23) | Interaction p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ% | ES | Pre | Post | Δ% | ES | Pre | Post | Δ% | ES | ||
| SMM (kg) | 17.7 ± 2.5 | 18.4 ± 2.4 *,§ | 3.4 ± 2.9 | 0.25 | 17.4 ± 3.2 | 18.2 ± 3.2 *,§ | 4.2 ± 2.3 | 0.23 | 16.2 ± 2.2 | 16.6 ± 2.2 * | 2.0 ± 2.1 | 0.14 | <0.001 |
| ULLST (kg) | 4.1 ± 0.47 | 4.2 ± 0.45 * | 3.4 ± 3.0 | 0.29 | 3.9 ± 0.54 | 4.1 ± 0.58 * | 5.9 ± 4.3 | 0.40 | 3.7 ± 0.53 | 3.9 ± 0.53 * | 4.1 ± 3.5 | 0.29 | 0.156 |
| LLLST (kg) | 11.8 ± 1.2 | 12.1 ± 1.1 *,§ | 3.2 ± 2.9 | 0.30 | 11.6 ± 1.4 | 12.0 ± 1.5 *,§ | 3.7 ± 2.2 | 0.30 | 11.3 ±1.4 | 11.4 ± 1.4 * | 1.1 ± 2.2 | 0.08 | <0.001 |
| CP (kg) | 46.0 ± 9.0 | 49.0 ± 10.0 *,§ | 5.6 ± 1.7 | 0.28 | 45.0 ± 9.0 | 48.0 ± 10.0 *,§ | 5.9 ± 1.6 | 0.28 | 43.0 ± 8.0 | 45.0 ± 8.0 * | 4.5 ± 1.2 | 0.24 | <0.05 |
| KE (kg) | 52.0 ± 11.0 | 56.0 ± 12.0 *,§ | 9.2 ± 2.5 | 0.39 | 55.0 ± 11.0 | 59.0 ± 12.0 *,§ | 8.8 ± 2.2 | 0.41 | 52.0 ± 13.0 | 56.0 ± 13.0 * | 7.5 ± 1.0 | 0.32 | <0.001 |
| PC (kg) | 23.0 ± 4.0 | 25.0 ± 4.0 * | 11.3 ± 5.7 | 0.59 | 22.0 ± 3.0 | 25.0 ± 4.0 * | 12.4 ± 6.6 | 0.75 | 21.0 ± 3.0 | 23.0 ± 4.0 * | 10.5 ± 5.3 | 0.63 | 0.376 |
| TS (kg) | 121.0 ± 20.0 | 131.0 ± 21.0 *,§ | 8.1 ± 1.6 | 0.48 | 122.0 ± 21.0 | 132.0 ± 22.0 *,§ | 8.3 ± 2.3 | 0.47 | 115.0 ± 21.0 | 124.0 ± 22.0 * | 7.0 ± 2.7 | 0.38 | <0.05 |
| Training load (kg) | 1735 ± 232 | 2505 ± 292 * | 45.3 ± 14.8 | 2.93 | 1698 ± 224 | 2429 ± 377 * | 43.6 ± 18.3 | 2.44 | 1630 ± 276 | 2367 ± 442 * | 44.7 ± 14.2 | 2.05 | 0.916 |
| 10 MW (s) | 7.5 ± 0.9 | 6.7 ± 0.9 *,§ | −10.8 ± 11.3 | 0.90 | 7.5 ± 1.0 | 6.6 ± 1.0 *,§ | −11.8 ± 8.6 | 0.89 | 7.3 ± 0.9 | 6.9 ± 0.7 * | −4.3 ± 8.4 | 0.41 | <0.05 |
| RSP (s) | 12.0 ± 1.6 | 10.8 ± 1.1 * | −10.0 ± 12.4 | 0.89 | 11.7 ± 1.5 | 10.5 ± 1.7 * | −10.1 ± 5.4 | 0.73 | 11.7 ± 1.8 | 11.0 ± 1.8 * | −5.7 ± 7.6 | 0.36 | 0.176 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B.; et al. Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial. Nutrients 2018, 10, 563. https://doi.org/10.3390/nu10050563
Nabuco HCG, Tomeleri CM, Sugihara Junior P, Fernandes RR, Cavalcante EF, Antunes M, Ribeiro AS, Teixeira DC, Silva AM, Sardinha LB, et al. Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial. Nutrients. 2018; 10(5):563. https://doi.org/10.3390/nu10050563
Chicago/Turabian StyleNabuco, Hellen C. G., Crisieli M. Tomeleri, Paulo Sugihara Junior, Rodrigo R. Fernandes, Edilaine F. Cavalcante, Melissa Antunes, Alex S. Ribeiro, Denilson C. Teixeira, Analiza M. Silva, Luís B. Sardinha, and et al. 2018. "Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial" Nutrients 10, no. 5: 563. https://doi.org/10.3390/nu10050563






