Surface Carbon Shell-Functionalized ZrO2 as Nanofiller in Polymer Gel Electrolyte-Based Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
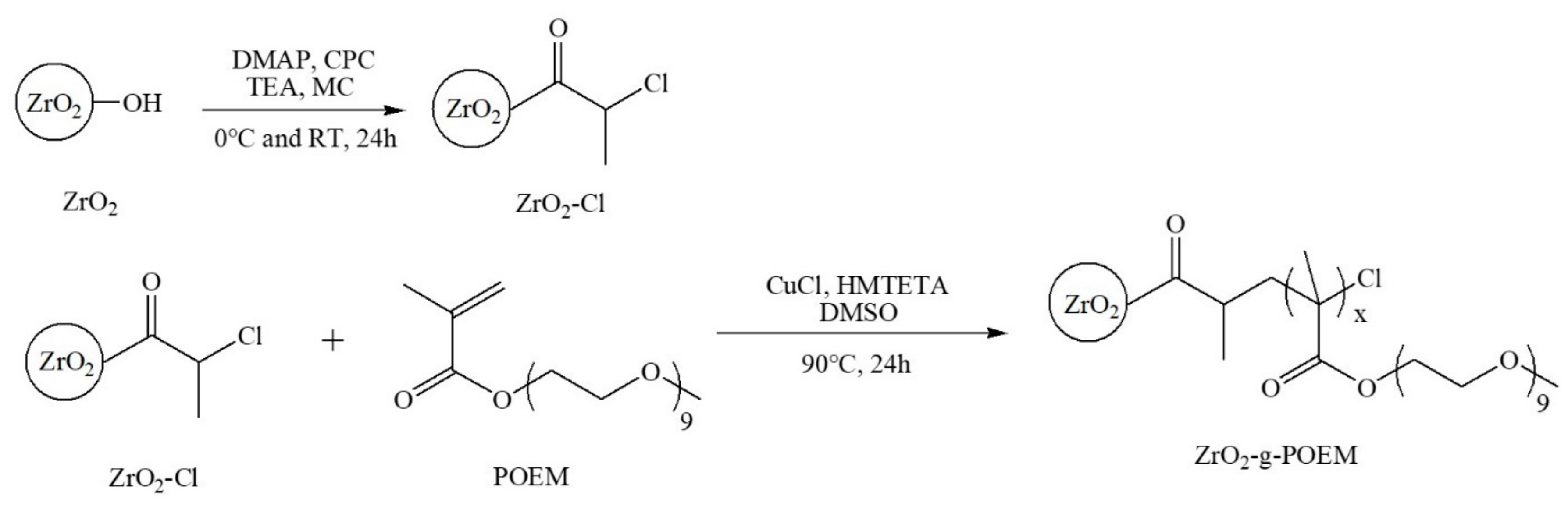
2.2. Surface Modification From ZrO2 to ZrO2-Cl
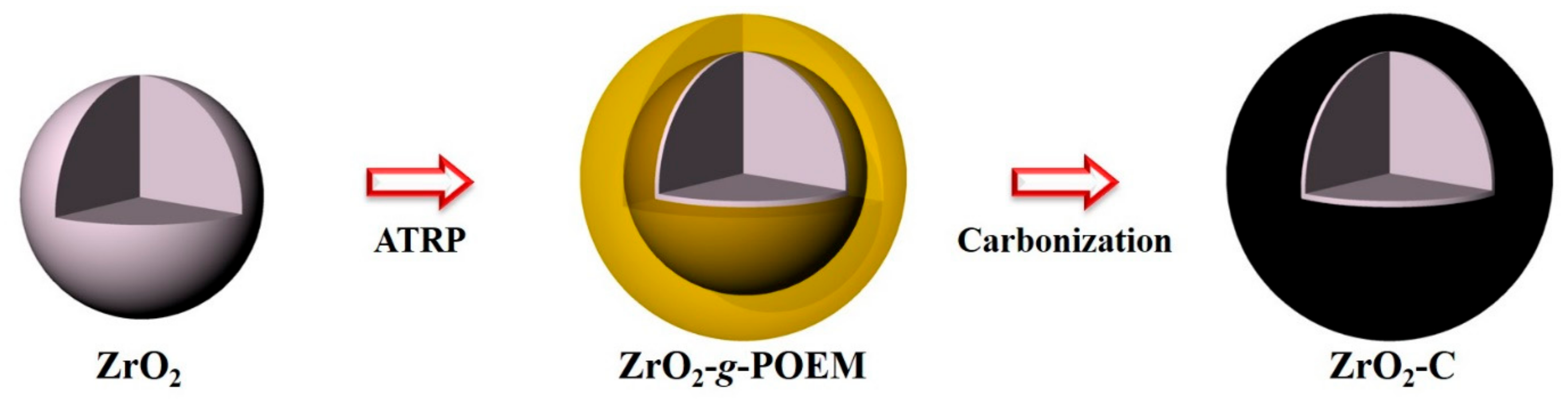
2.3. Synthesis of ZrO2-g-POEM via ATRP
2.4. Preparation of ZrO2-C
2.5. Preparation of ZrO2-C/PGEs
2.6. Fabrication of ZrO2-C/PGE-Based DSSCs
2.7. Characterization
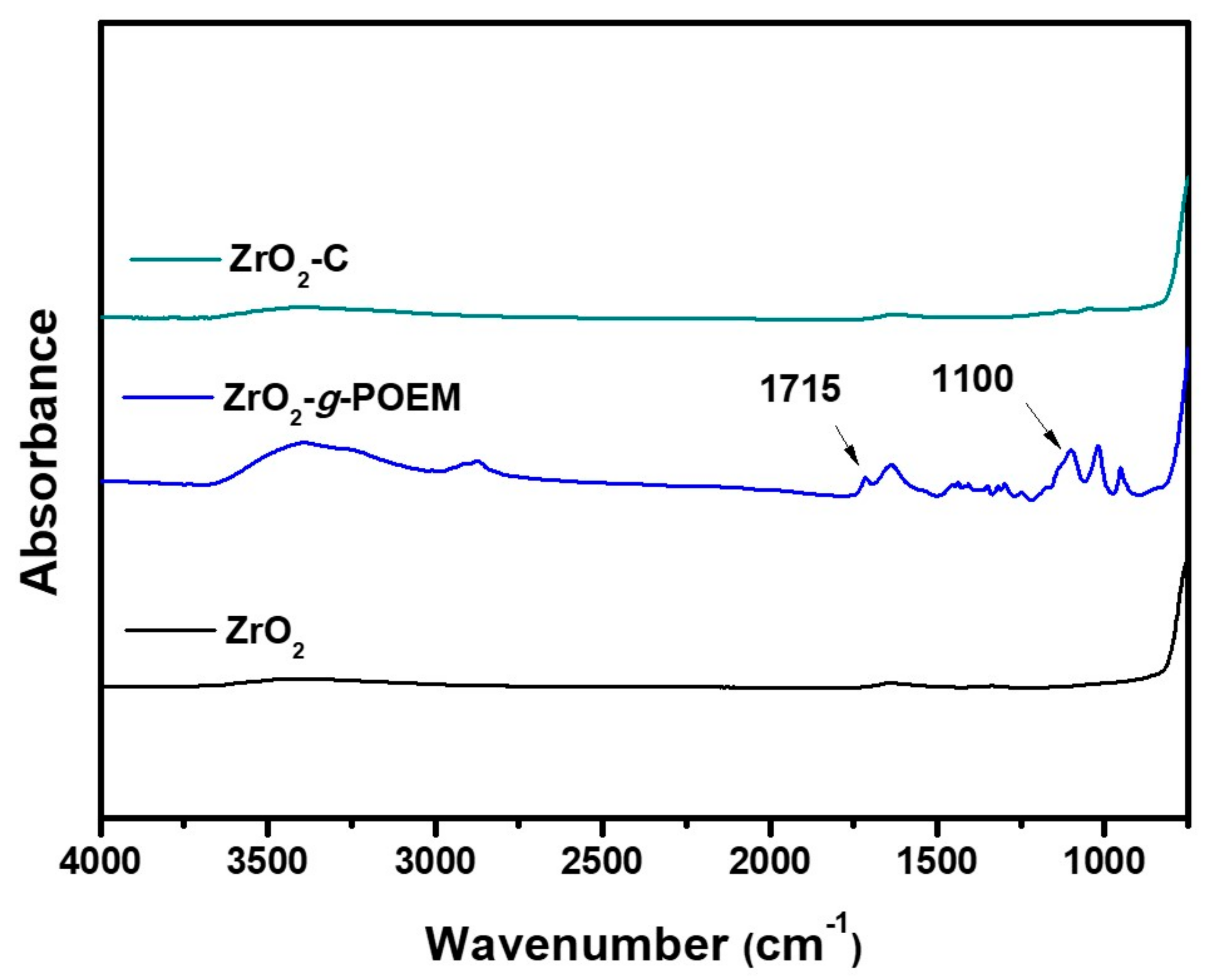
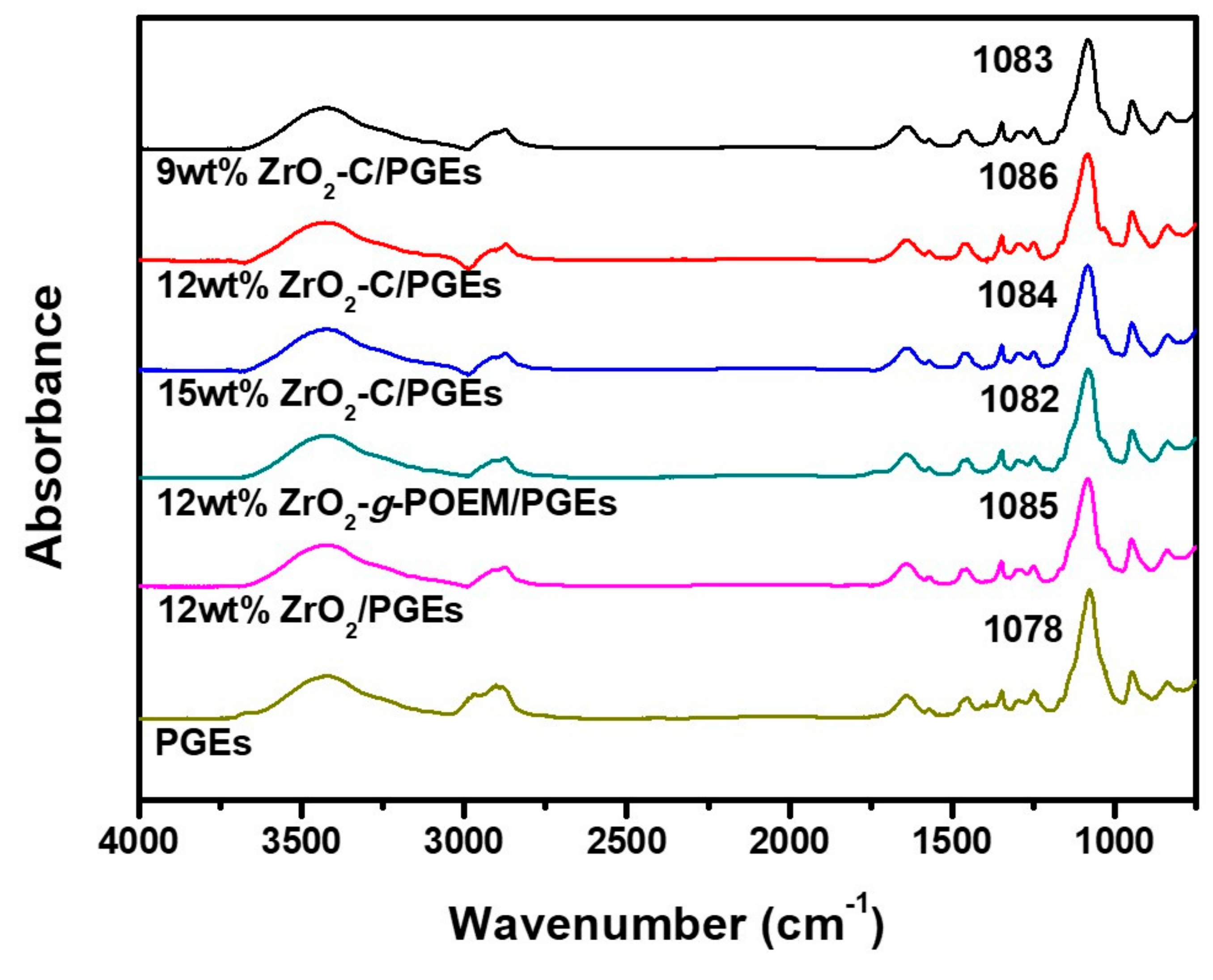
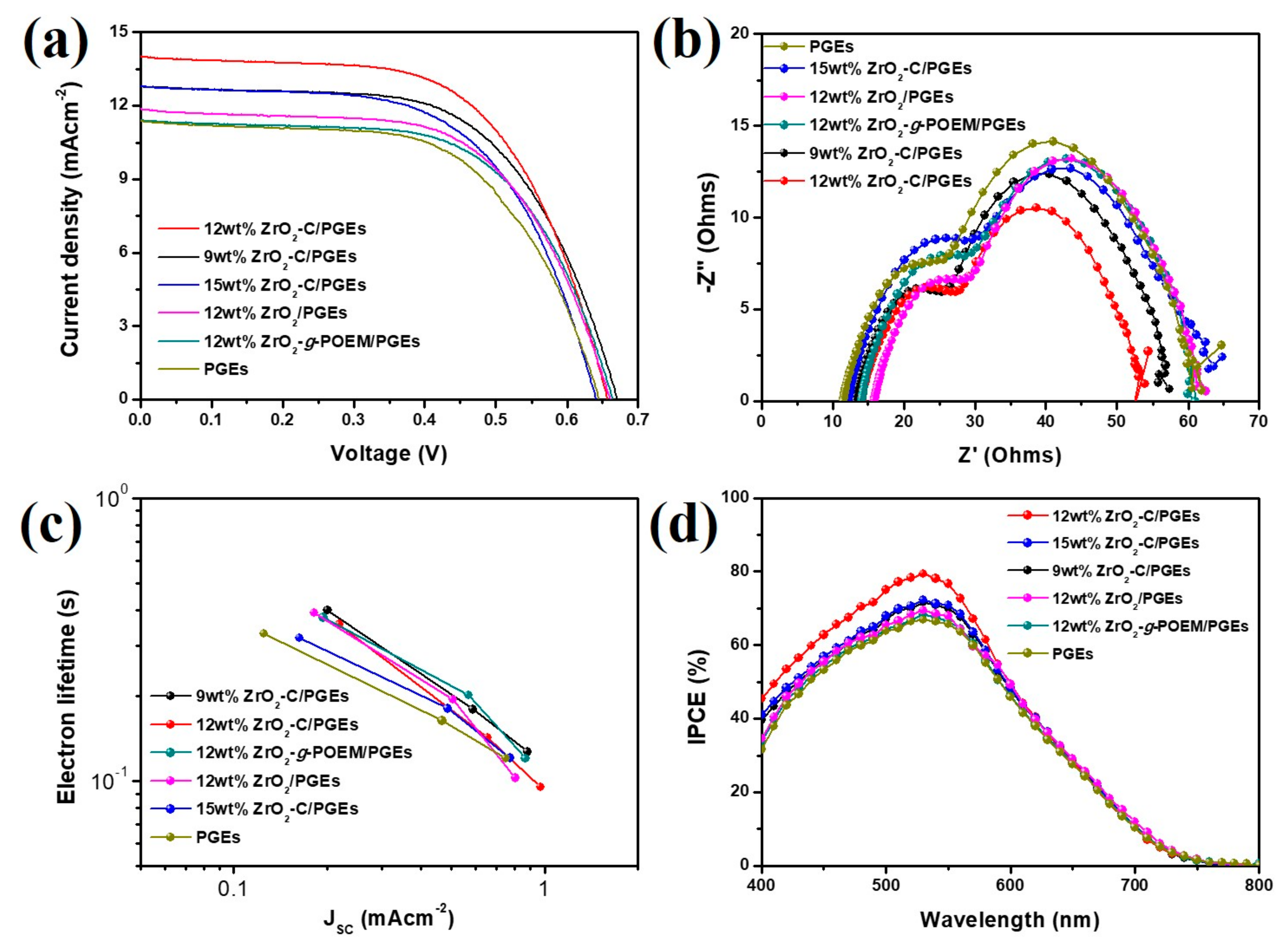
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, F.; Ou, Y.; Liu, G.; Zhao, L.; Dong, B.; Wang, S.; Wen, S. Novel Quasi-Solid-State Electrolytes based on Electrospun Poly(vinylidene fluoride) Fiber Membranes for Highly Efficient and Stable dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zakeeruddin, S.M.; Exnar, I.; Grätzel, M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem. Commun. 2002, 24, 2972–2973. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, M.; Wu, J.; Lan, Z.; Hao, S.; Lin, J. The polymer gel electrolyte based on poly(methyl methacrylate) and its application in quasi-solid-state dye-sensitized solar cells. Mater. Chem. Phys. 2008, 110, 38–42. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Wang, Y.; Travas-Sejdic, J.; Steiner, R. Polymer gel electrolyte supported with microporous polyolefin membranes for lithium ion polymer battery. Solid State Ion. 2002, 7, 443–449. [Google Scholar] [CrossRef]
- Rao, M.M.; Liu, J.S.; Li, W.S.; Liang, Y.; Zhou, D.Y. Preparation and performance analysis of PE-supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery application. J. Membr. Sci. 2008, 322, 314–319. [Google Scholar] [CrossRef]
- Shin, W.K.; Cho, J.; Kannan, A.G.; Lee, Y.S.; Kim, D.W. Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef]
- Łatoszyńska, A.A.; Taberna, P.L.; Simon, P.; Wieczorek, W. Proton conducting Gel Polymer Electrolytes for supercapacitor applications. Electrochim. Acta 2017, 242, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Suzuka, M.; Hayashi, N.; Sekiguchi, T.; Sumioka, K.; Takata, M.; Hayo, N.; Ikeda, H.; Oyaizu, K.; Nishide, H.A. Quasi-Solid State DSSC with 10.1% Efficiency through Molecular Design of the Charge-Separation and-Transport. Sci. Rep. 2016, 6, 28022. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef]
- George, J.; Ishida, H. A review on the very high nanofiller-content nanocomposites: Their preparation methods and properties with high aspect ratio fillers. Prog. Polym. Sci. 2018, 86, 1–39. [Google Scholar] [CrossRef]
- Lei, X.X.; Lu, H.; Lu, L.; Xu, H.Q.; Zhou, Y.G.; Zou, J. Improving the Thermal and Mechanical Properties of Poly(l-lactide) by Forming Nanocomposites with an in Situ Ring-Opening Intermediate of Poly(l-lactide) and Polyhedral Oligomeric Silsesquioxane. Nanomaterials 2019, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Ningaraju, S.; Gnana Prakash, A.P.; Ravikumar, H.B. Studies on free volume controlled electrical properties of PVA/NiO and PVA/TiO2 polymer nanocomposites. Solid State Ion. 2018, 320, 132–147. [Google Scholar] [CrossRef]
- Fan, L.W.; Fang, X.; Wang, X.; Zeng, Y.; Xiao, Y.Q.; Yu, Z.T.; Xu, X.; Hu, Y.C.; Cen, K.F. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. [Google Scholar] [CrossRef]
- Ningaraju, S.; Hegde, V.N.; Prakash, A.P.G.; Ravikumar, H.B. Free volume dependence on electrical properties of Poly (styrene co-acrylonitrile)/Nickel oxide polymer nanocomposites. Chem. Phys. Lett. 2018, 698, 24–35. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mallakpour, S.; Borandeh, S. Improving interfacial interaction of l-phenylalanine-functionalized graphene nanofiller and poly(vinyl alcohol) nanocomposites for obtaining significant membrane properties: Morphology, thermal, and mechanical studies. Polym. Compos. 2016, 37, 1924–1935. [Google Scholar] [CrossRef]
- Mohan, V.M.; Murakami, K.; Kono, A.; Shimomura, M. Poly(acrylonitrile)/activated carbon composite polymer gel electrolyte for high efficiency dye sensitized solar cells. J. Mater. Chem. A 2013, 1, 7399–7407. [Google Scholar] [CrossRef]
- Lee, C.P.; Chen, P.Y.; Vittal, R.; Ho, K.C. Iodine-free high efficient quasi solid-state dye-sensitized solar cell containing ionic liquid and polyaniline-loaded carbon black. J. Mater. Chem. 2010, 20, 2356–2361. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lee, C.P.; Vittal, R.; Ho, K.C. A quasi solid-state dye-sensitized solar cell containing binary ionic liquid and polyaniline-loaded carbon black. J. Power Sources 2010, 195, 3933–3938. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Ehsani, A.; Bigdeloo, M.; Ansari, M.Y.; Mirtamizdoust, B.; Heidari, A.A.; Hadi, M.; Shiri, H.M. Nanocomposite of Conjugated Polymer/Nano-Flowers Cu(II) Metal-Organic System with 2-Methylpyridinecarboxaldehyde Isonicotinohydrazide as a Novel and Hybrid Electrode Material for Highly Capacitive Pseudocapacitors. Bull. Chem. Soc. Jpn. 2018, 91, 617–622. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Park, J.T.; Lee, C.S.; Park, C.H.; Kim, J.H. Preparation of TiO2/Ag binary nanocomposite as high-activity visible-light-driven photocatalyst via graft polymerization. Chem. Phys. Lett. 2017, 685, 119–126. [Google Scholar] [CrossRef]
- Ergun, G.; Sahin, Z.; Ataol, A.S. The effects of adding various ratios of zirconium oxide nanoparticles to poly(methyl methacrylate) on physical and mechanical properties. J. Oral Sci. 2018, 60, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, P.; Choudhary, R.B.; Majhi, M. Structural, optical and dielectric properties of ZrO2 reinforced polymeric nanocomposite films of polymethylmethacrylate (PMMA). Optik 2016, 127, 4848–4853. [Google Scholar] [CrossRef]
- Solarajan, A.K.; Murugadoss, V.; Angaiah, S. Dimensional stability and electrochemical behaviour of ZrO2 incorporated electrospun PVdF-HFP based nanocomposite polymer membrane electrolyte for Li-ion capacitors. Sci. Rep. 2017, 7, 45390. [Google Scholar] [CrossRef]
- Park, J.T.; Chi, W.S.; Roh, D.K.; Ahn, S.H.; Kim, J.H. Hybrid Templated Synthesis of Crack-Free, Organized Mesoporous TiO2 Electrodes for High Efficiency Solid-State Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2013, 23, 26–33. [Google Scholar] [CrossRef]
- Park, J.T.; Chi, W.S.; Jeon, H.; Kim, J.H. Improved electron transfer and plasmonic effect in dye-sensitized solar cells with bi-functional Nb-doped TiO2/Ag ternary nanostructures. Nanoscale 2014, 6, 2718–2729. [Google Scholar] [CrossRef]
- Heo, S.Y.; Koh, J.K.; Kang, G.; Ahn, S.H.; Chi, W.S.; Kim, K.; Kim, J.H. Bifunctional Moth-Eye Nanopatterned Dye-Sensitized Solar Cells: Light-Harvesting and Self-Cleaning Effects. Adv. Energy Mater. 2014, 4, 1300632. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization: From Mechanisms to Applications. Isr. J. Chem. 2012, 52, 206–220. [Google Scholar] [CrossRef]
- Kolvari, E.; Koukabi, N.; Hosseini, M.M.; Vahidian, M.; Ghobadi, E. Nano-ZrO2 sulfuric acid: Aheterogeneous solid acid nano catalyst for Biginelli reaction under solvent free conditions. RSC Adv. 2016, 6, 7419–7425. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lei, B.X.; Fang, W.J.; Hou, Y.F.; Liao, J.Y.; Kuang, D.B.; Su, C.Y. All-solid-state electrolytes consisting of ionic liquid and carbon black for efficient dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2010, 216, 8–14. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Jayathissa, R.; Seneviratne, V.A.; Thotawatthage, C.A.; Senadeera, G.K.R.; Mellander, B.E. Polymethylmethacrylate (PMMA) based quasi-solid electrolyte with binary iodide salt for efficiency enhancement in TiO2 based dye sensitized solar cells. Solid State Ion. 2014, 265, 85–91. [Google Scholar] [CrossRef]
- Suresh, T.; Joseph, J.; Son, K.M.; Vittal, R.; Lee, J.; Kim, K.J. Effects of pH of a hybrid gel incorporated with 3-aminopropyltrimethoxysilane on the performance of a quasi-solid state dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 2007, 91, 1313–1318. [Google Scholar] [CrossRef]
- Chae, H.; Song, D.; Lee, Y.G.; Son, T.; Cho, W.; Pyun, Y.B.; Kim, T.Y.; Lee, J.H.; Fabregat-Santiago, F.; Bisquert, J.; et al. Chemical Effects of Tin Oxide Nanoparticles in Polymer Electrolytes-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 16510–16517. [Google Scholar] [CrossRef]
- Park, J.T.; Ahn, S.H.; Roh, D.K.; Lee, C.S.; Kim, J.H. Multifunctional Organized Mesoporous Tin Oxide Films Templated by Graft Copolymers for Dye-Sensitized Solar Cells. ChemSusChem 2014, 7, 2037–2047. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017, 46, 5975–6023. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Datta, J. CdTe nanoparticles decorated titania for dye sensitized solar cell: A novel co-sensitizer approach towards highly efficient energy conversion. New J. Chem. 2017, 41, 8663–8672. [Google Scholar] [CrossRef]
- Tang, B.; Yu, H.; Huang, W.; Sun, Y.; Li, X.; Li, S.; Ma, T. Three-dimensional graphene networks and RGO-based counter electrode for DSSCs. RSC Adv. 2019, 9, 15678–15685. [Google Scholar] [CrossRef] [Green Version]


| Electrolyte | Voc (V) | Jsc (mA/cm2) | FF | η (%) | RS | R-CE | R-TiO2 | WS |
|---|---|---|---|---|---|---|---|---|
| 9 wt% ZrO2-C/PGEs | 0.67 | 12.8 | 0.61 | 5.2 | 13.1 | 12.8 | 30.4 | 1.4 |
| 12 wt% ZrO2-C/PGEs | 0.66 | 14.0 | 0.61 | 5.6 | 14.1 | 12.6 | 26 | 1.2 |
| 15 wt% ZrO2-C/PGEs | 0.64 | 12.8 | 0.60 | 4.9 | 12.2 | 16.4 | 32.6 | 3.0 |
| 12 wt% ZrO2-g-POEM/PGEs | 0.67 | 11.5 | 0.61 | 4.7 | 14.0 | 14.5 | 30.6 | 1.7 |
| 12 wt% ZrO2/PGEs | 0.66 | 11.9 | 0.61 | 4.8 | 15.8 | 13.6 | 31 | 2 |
| PGEs | 0.64 | 11.4 | 0.60 | 4.4 | 11.4 | 14.2 | 34.5 | 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.M.; Moon, J.; Choi, G.H.; Baek, U.C.; Lim, J.M.; Park, J.T.; Kim, J.H. Surface Carbon Shell-Functionalized ZrO2 as Nanofiller in Polymer Gel Electrolyte-Based Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 1418. https://doi.org/10.3390/nano9101418
Lim SM, Moon J, Choi GH, Baek UC, Lim JM, Park JT, Kim JH. Surface Carbon Shell-Functionalized ZrO2 as Nanofiller in Polymer Gel Electrolyte-Based Dye-Sensitized Solar Cells. Nanomaterials. 2019; 9(10):1418. https://doi.org/10.3390/nano9101418
Chicago/Turabian StyleLim, Seung Man, Juyoung Moon, Gyo Hun Choi, Uoon Chul Baek, Jeong Min Lim, Jung Tae Park, and Jong Hak Kim. 2019. "Surface Carbon Shell-Functionalized ZrO2 as Nanofiller in Polymer Gel Electrolyte-Based Dye-Sensitized Solar Cells" Nanomaterials 9, no. 10: 1418. https://doi.org/10.3390/nano9101418





