Antibacterial Application on Staphylococcus aureus Using Antibiotic Agent/Zinc Oxide Nanorod Arrays/Polyethylethylketone Composite Samples
Abstract
:1. Introduction
2. Materials and Methods
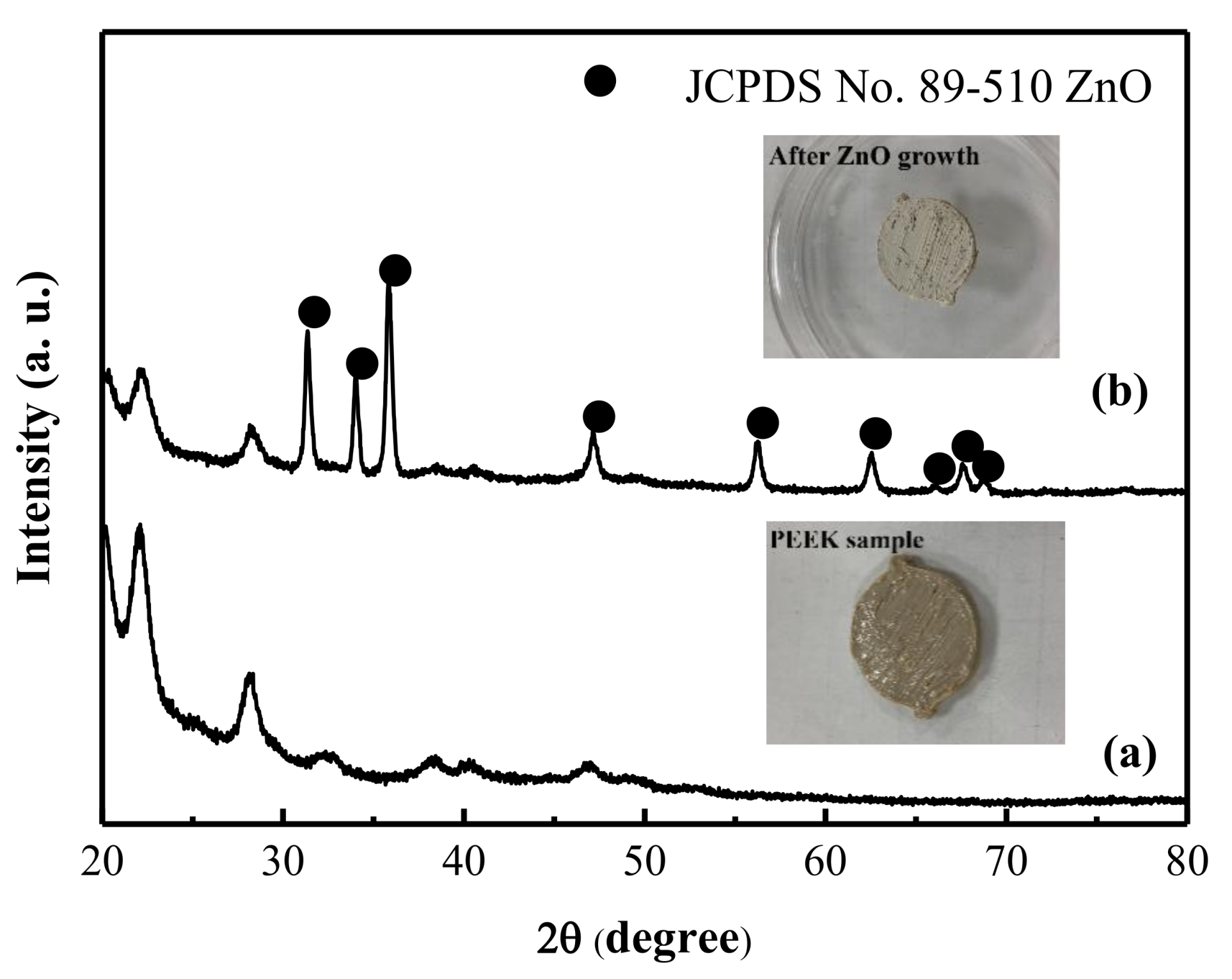
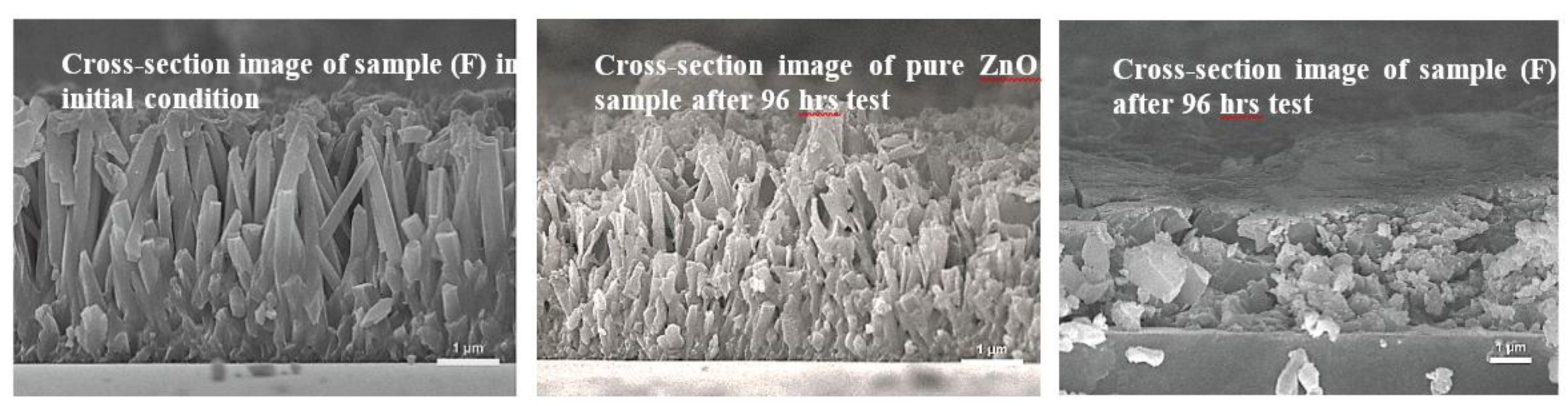
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quyang, L.; Zhao, Y.; Jin, G.; Lu, T.; Li, J.; Qiao, Y.; Ning, C.; Zhang, X.; Chu, P.K.; Liu, X. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials 2017, 83, 115–126. [Google Scholar]
- Hasan, J.; Crawford, R.; Ivanova, E. P Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Tang, Z.K.; Wong, G.K.L.; Yu, P.; Kawasaki, M.; Ohtomo, A.; Koinuma, H.; Segawa, Y. Room-temperature ultraviolet laser emission from self-assembled ZnO microcrystallite thin films. Appl. Phys. Lett. 1998, 72, 3270–3272. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Li, Q.K.; Nan, C.W. Synthesis and optical properties of tetrapod-like zinc oxide nanorods. Chem. Phys. Lett. 2002, 358, 83–86. [Google Scholar] [CrossRef]
- Song, J.; Lim, S. Effect of seed layer on the growth of ZnO nanorods. J. Phys. Chem. C 2007, 111, 596–600. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Summers, C.J.; Wang, Z.L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Feng, X.; Lu, J.; Zheng, Y.; Wang, X.; Volinsky, A.A.; Wang, L.N. Antibacterial activity and inflammation inhibition of ZnO nanoparticles embedded TiO2 nanotubes. Nanotechnology 2018, 29, 244003. [Google Scholar] [CrossRef] [PubMed]
- Ipeksac, T.; Kaya, F.; Kaya, C. Hydrothermal synthesis of zinc oxide (ZnO) nanotubes and its electrophoretic deposition on nickel filter. Mater. Lett. 2013, 100, 11–14. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Lewandowska, M. Synthesis and characterization of ZnO and Ag nanoparticle-load TiO2 nanotube composite layers intended for antibacterial coating. Thin Solid Films 2014, 553, 173–178. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhang, D. A novel multifunctional electrochemical platform for simultaneous detection, elimination, and inactivation of pathogenic bacterial based on the vancomycin-function Ag-NPs/3D ZnO nanorod arrays. Biosens. Bioelectron. 2017, 98, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Sr/ZnO doped titania nanotube arrays: An effective surface system with excellent osteoinductivity and self-antibacterial activity. Mater. Des. 2017, 130, 403–412. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2017, 97, 629–637. [Google Scholar] [CrossRef]
- Agnihotri, S.; Bajaj, G.; Mukherji, S.; Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: An enhanced and reusable antibacterial substrates with human cell cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Holloysite nanotubes supported Ag and ZnO nanoparticles with synergistically enhanced antibacterial activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef]
- Liu, W.; Su, P.; Gonzales, A.; Chen, S.; Wang, N.; Wang, J.; Li, H.; Zhang, Z.; Webster, T.J. Optimizing stem cell functions and antibacterial properties of TiO2 nanotubes incorporated with ZnO nanoparticles: Experiments and modelling. Int. J. Nanomed. 2015, 10, 1997–2019. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Clare-Salzler, Z.J.; Zaveri, T.D.; Mehta, S.; Dolgova, N.V.; Chu, B.H.; Ren, F.; Keselowsky, B.G. Antibacterial effects of zinc oxide nanorod surfaces. J. Nanosci. Nanotechnol. 2012, 12, 7131–7138. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Jeong, Y.S.; Anwar, M.S.; Dwivedi, S.; Alsharaeh, E.; Koo, B.H. Novel biomimetic synthesis of ZnO nanorods using egg white(albumen) and their antibacterial studies. J. Nanosci. Nanotechnol. 2016, 16, 5959–5965. [Google Scholar] [CrossRef]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Marti, M.; Pividon, M.I.; Simonelli, G.; et al. Control degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C 2018, 93, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, S.; Sundar, J.K. Investigations of visible light driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Appl. Surf. Sci. 2018, 449, 617–630. [Google Scholar]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticels against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4427–4439. [Google Scholar] [CrossRef]
- Jana, T.K.; Jana, S.K.; Kumar, A.; De, K.; Maiti, R.; Mandal, A.K.; Chatterjee, T.; Chatterjee, B.K.; Charrabarti, P.; Chatterjee, K. The antibacterial and anticancer properties of zinc oxide coated iron oxide nanotextured composites. Colloides Surf. B Biointerfaces 2019, 177, 512–519. [Google Scholar] [CrossRef]
- Singh, R.; Verma, K.; Patyal, A.; Sharma, I.; Barman, P.B.; Sharma, D. Nanosheet and nanosphere morphology dominated photocatalytic & antibacterial properties of ZnO nanostructures. Solid State Sci. 2018, 89, 1–14. [Google Scholar]
- Chen, J.H.; Yao, Y.Y.; Tang, H.C.; Lin, T.Y.; Chen, D.W.; Cheng, K.W. Long-term antibacterial performances of biodegradation polylactic acid materials with directly absorption of antibiotic agents. RSC Adv. 2018, 8, 16223–16231. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Fu, C.; Yang, F.; Jiao, Z.; Shi, X.; Ito, Y.; Wang, Z.; Liu, Q.; Zhang, P. Micro-porous polyetherehterketone implants decorated with BMP-2 via phosphorylated gelatin coating for enhance cell adhesion and osteogenic differentiation. Colloids Surf. B Biointerfaces 2018, 169, 233–241. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [Green Version]
- Duraimurugan, J.; Suresh Kumar, G.; Venkatesh, M.; Maadeswaran, P.; Girija, E.K. Morphology and size controlled synthesis of zinc oxide nanostructures and their optical properties. J. Mater. Sci. Mater. Electron. 2018, 29, 9339–9346. [Google Scholar] [CrossRef]
- Lee, W.; Leem, J.Y. Size control of ZnO nanorods using the hydrothermal method in conjunction with substrate rotation. J. Nanosci. Nanotechnol. 2017, 17, 7952–7956. [Google Scholar] [CrossRef]
- Kokotov, M.; Hodes, G. Reliable chemical bath deposition of ZnO films with controllable morphology from ethanolamine-based solutions using KMnO4 substrate activation. J. Mater. Chem. 2009, 19, 3847–3854. [Google Scholar] [CrossRef]
- Tak, Y.; Yong, K. Controlled growth of well-aligned ZnO nanorod array using a novel solution method. J. Phys. Chem. B 2005, 109, 19263–19269. [Google Scholar] [CrossRef]
- Arnold, M.S.; Avouris, P.; Pan, Z.W.; Wang, Z.L. Field-Effect transistors based on single semiconducting oxide nanobelts. J. Phys. Chem. B 2003, 107, 659–663. [Google Scholar] [CrossRef]
- Vallejos, S.; Gràcia, I.; Figueras, E.J.; Prášek, J.; Sedláček, J.; Pytlíček, Z.; Cané, C. Ferric oxide nanoparticle-functionalized tungsten oxide nanoneedles and their gas sensing properties. Procedia Eng. 2015, 120, 443–446. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]
- Kang, M.A.; Kang, J.S. Stability test of ampicillin sodium solutions in the accufuser® elastomeric infusion device Using HPLC: UV method. Pharmacol. Pharm. 2012, 4, 462–467. [Google Scholar] [CrossRef]
- Čepin, M.; Jovanovski, V.; Podlogar, M.; Orel, Z.C. Amino- and ionic liquid-functionalised nanocrystalline ZnO via silane anchoring—An antimicrobial synergy. J. Mater. Chem. B 2015, 3, 1059–1067. [Google Scholar] [CrossRef]
- Malzahn, K.; Jamieson, W.D.; Dröge, M.; Mailänder, V.; Jenkins, A.T.A.; Weiss, C.K.; Landfester, K. Advanced dextran based nanogels for fighting Staphylococcus aureus infections by sustained zinc release. J. Mater. Chem. B 2014, 2, 2175–2183. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.H.; Gao, G.; Chen, X.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl. Mater. Int. 2016, 8, 32170–32181. [Google Scholar] [CrossRef]

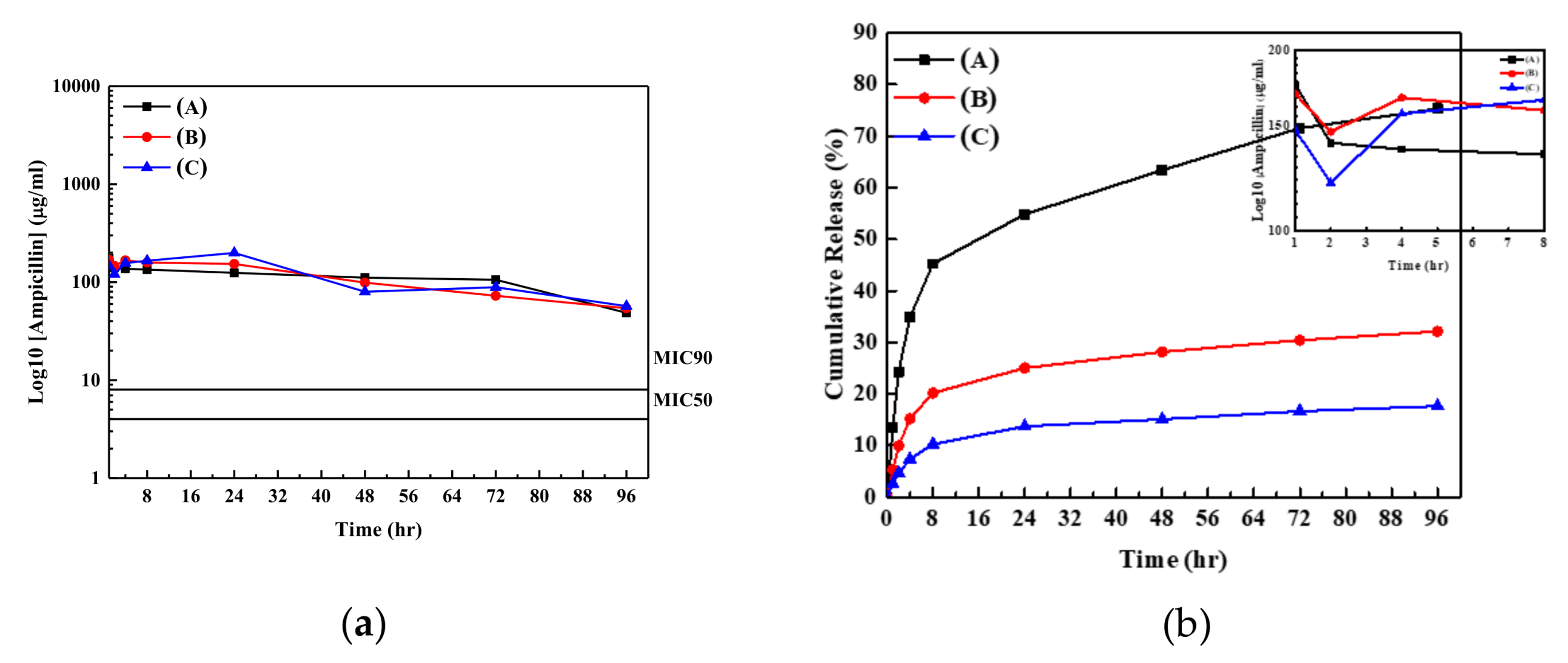
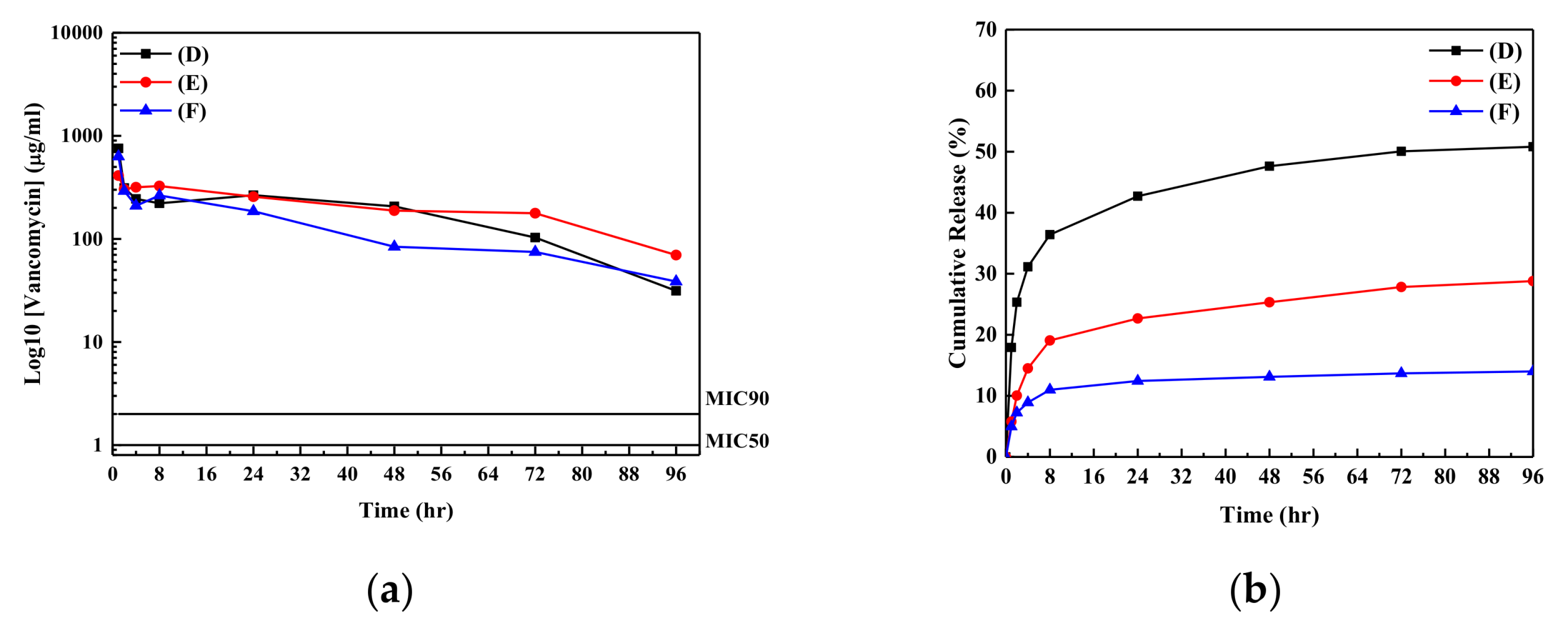


| Sample | Antibiotic Agent for Absorption | Concentration of Antibiotic Agent for Absorption | Absorption Percentage (%) | Rate Constant for K-P Model (hr−1) | n Values for the K-P Model | Value of R2 |
|---|---|---|---|---|---|---|
| (A) | Ampicillin | 5 mg/mL | 17.4 | 9.05 | 0.27 | 0.97 |
| (B) | Ampicillin | 10 mg/mL | 21.1 | 6.58 | 0.28 | 0.95 |
| (C) | Ampicillin | 15 mg/mL | 25.6 | 4.35 | 0.32 | 0.94 |
| (D) | Vancomycin | 5 mg/mL | 56.1 | 9.22 | 0.17 | 0.98 |
| (E) | Vancomycin | 10 mg/mL | 47.5 | 6.61 | 0.25 | 0.94 |
| (F) | Vancomycin | 15 mg/mL | 56.4 | 5.68 | 0.16 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.W.; Lee, K.-Y.; Tsai, M.-H.; Lin, T.-Y.; Chen, C.-H.; Cheng, K.-W. Antibacterial Application on Staphylococcus aureus Using Antibiotic Agent/Zinc Oxide Nanorod Arrays/Polyethylethylketone Composite Samples. Nanomaterials 2019, 9, 713. https://doi.org/10.3390/nano9050713
Chen DW, Lee K-Y, Tsai M-H, Lin T-Y, Chen C-H, Cheng K-W. Antibacterial Application on Staphylococcus aureus Using Antibiotic Agent/Zinc Oxide Nanorod Arrays/Polyethylethylketone Composite Samples. Nanomaterials. 2019; 9(5):713. https://doi.org/10.3390/nano9050713
Chicago/Turabian StyleChen, Dave W., Kuan-Yi Lee, Min-Hua Tsai, Tung-Yi Lin, Chien-Hao Chen, and Kong-Wei Cheng. 2019. "Antibacterial Application on Staphylococcus aureus Using Antibiotic Agent/Zinc Oxide Nanorod Arrays/Polyethylethylketone Composite Samples" Nanomaterials 9, no. 5: 713. https://doi.org/10.3390/nano9050713




