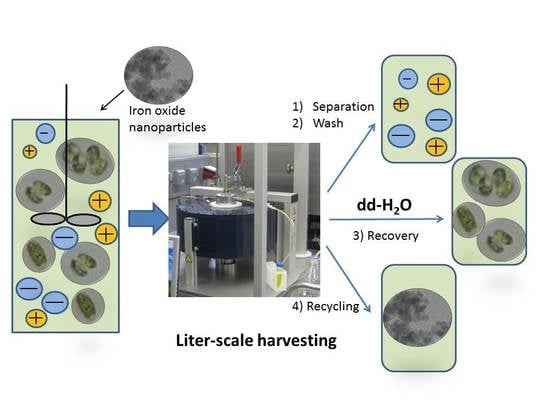
Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization
Abstract
:1. Introduction
2. Materials and Methods
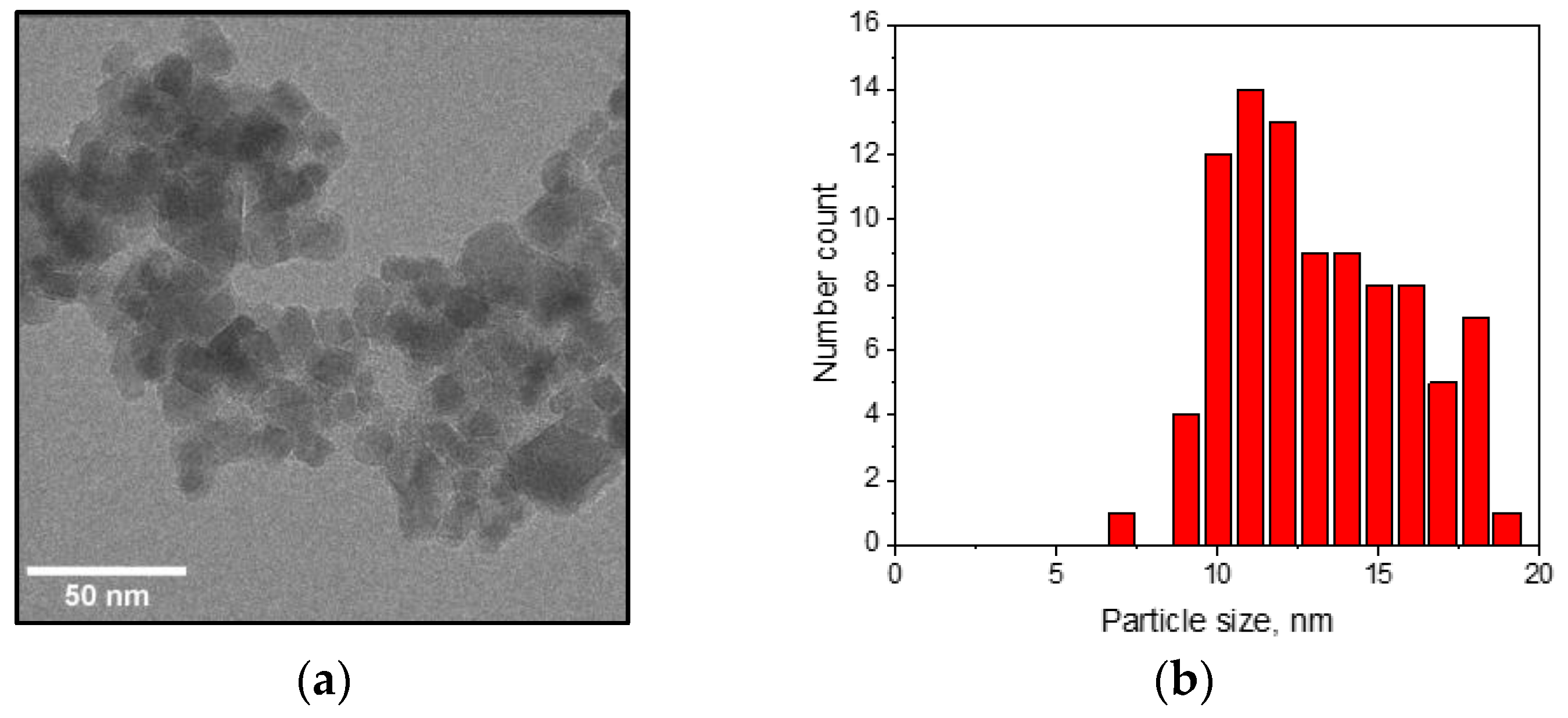
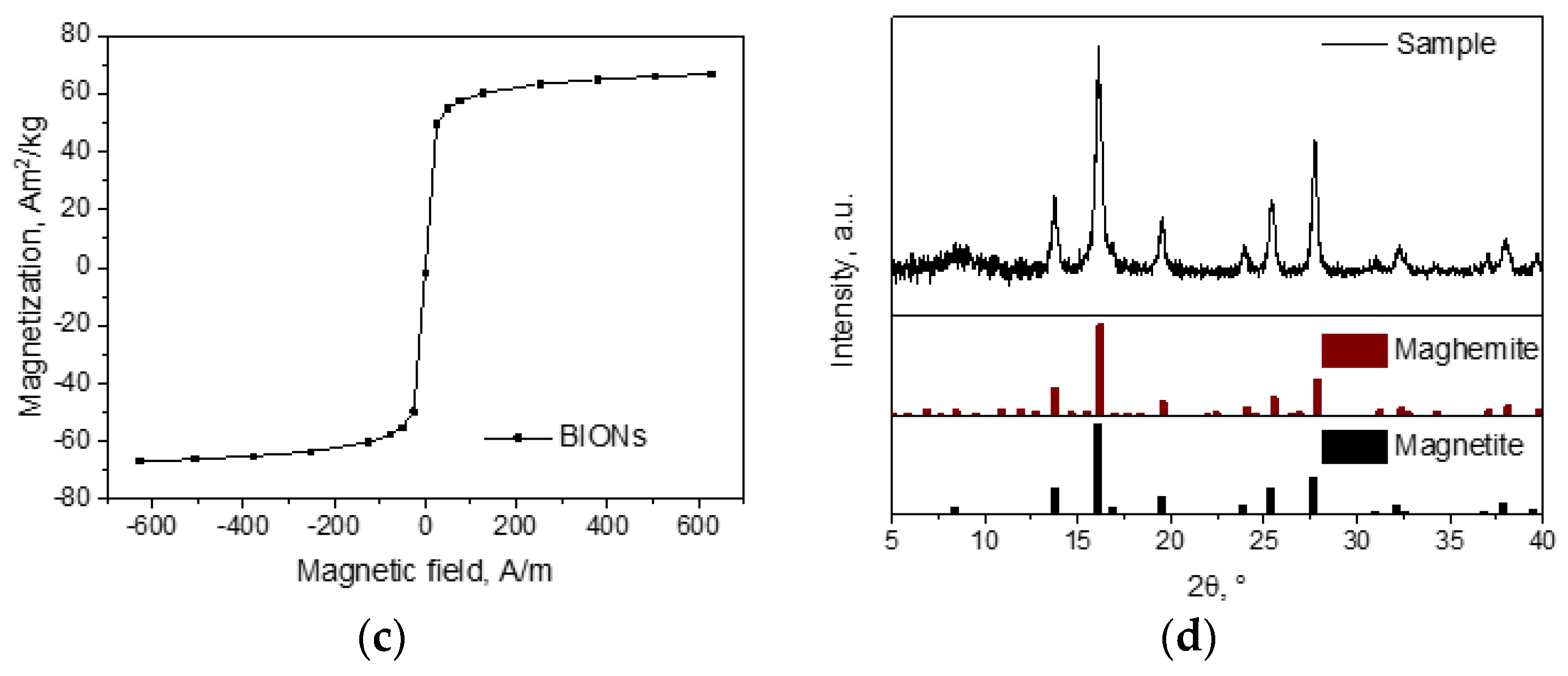
2.1. Nanoparticle Synthesis and Characterization
2.2. Microalgae
2.3. Methods and Instrumentation for Interaction Experiments
2.4. High-Gradient Magnetic Separation
- (1)
- The adsorption step, which takes place in an external vessel. BIONs and algae are incubated through stirring for 5 min at approximately 200 min−1 (RZR 2051, Heidolph Instruments, Schwabach, Germany) and yield a final suspension which is the feedstock for the HGMS process.
- (2)
- The separation step, in which the algae are separated from the broth. While the feedstock flows through the magnetic filter, the electromagnetic field is switched on, enabling the retention of the algae attached to the BIONs inside the filter. At the same time, the medium flows out of the separator.
- (3)
- Wash steps, in which the removal of weakly bound biomaterial and salt takes place. In this phase, a water solution rinses the filter chamber in a closed loop with the rotor on. The magnetic field is still switched on to retain the nanoparticles with the attached biomass, whereas the wash buffer is drained from the separator and collected in an external vessel.
- (4)
- Recovery steps, in which detachment of the microalgae takes place. This is achieved by again pumping water through the filter chamber in a closed loop after resuspending the BIONs with the magnetic field switched off and the stirrer switched on. Afterwards, the magnetic field is switched on again to separate BIONs from the eluted volume. The eluted fractions with the microalgae are collected in an external vessel.
- (5)
- The recycling step, in which the washed BIONs are recovered externally for further application. This step works similar to (4) and leads to a resuspension of the magnetic carrier (magnetic field off and stirrer on).
3. Results
3.1. Characterization of the Nanoparticles
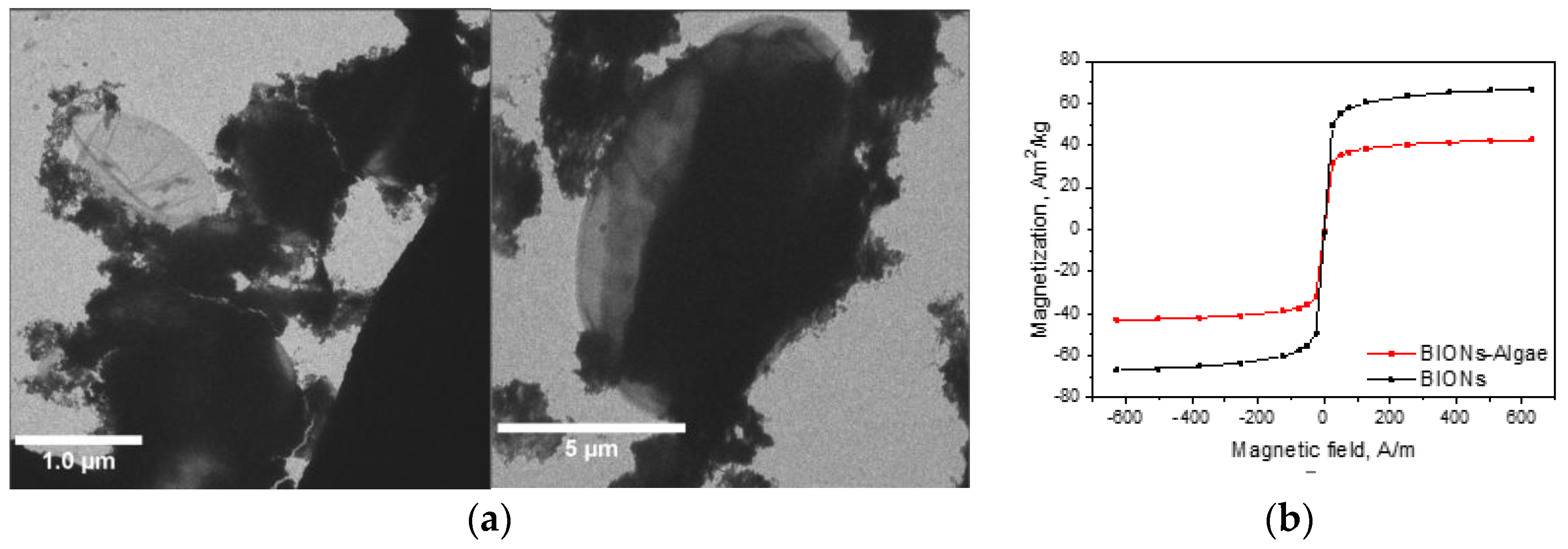
3.2. Interaction of the Microalgae with the BIONs
3.2.1. Adhesion Behavior
3.2.2. Microalgae Recovery
3.3. Upscaled Processing Using High-Gradient Magnetic Separation
4. Discussion
- -
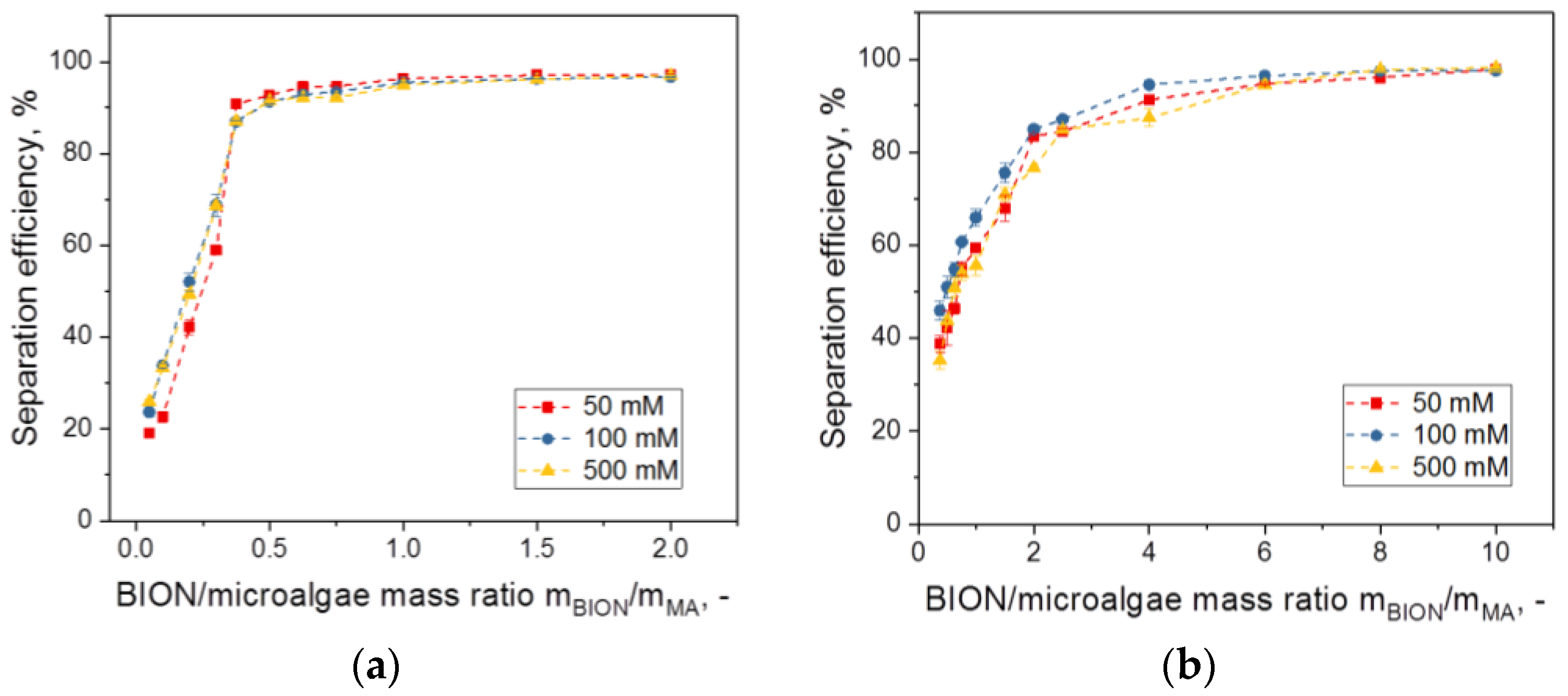
- For values above a threshold (saturation value) of the mass ratio of the nanoparticles to the microalgae, the separation efficiency of the microalgae comes close to 100%. The slope and saturation values of the separation efficiency diagram are specific for each system and depend on surface composition, dimensions, and the morphology of the microalgae species as well as of the nanoparticles and the content of the aqueous phase.
- -
- The separation efficiency decreases with increasing pH. However, with a sufficient mass of magnetic material, separation is possible in a very broad pH window. The results of Xu et al. with Chlorella ellipsoidea showed a parabolic behavior of the efficiency with the pH with the highest value at neutral pH, but these results represent an exception [37].
- -
- Although in our experiments no dependence was recognizable for NaCl concentrations between 0.05 and 0.5 M, this is probably not a general rule for all salts but depends on the salt and the concentration range. That the impact of ionic strength on separation depends on the salt selected was demonstrated by Procházková, who showed that phosphates have a strong effect and lead to a decreasing separation efficiency, whereas other anions did not [40]. We have also illustrated the high affinity of phosphate or citrate ions for the BION surface in previous work on adsorption of peptides and proteins [57], which explains the lower adsorption of other substances to the surface. Further adhesion experiments should deepen these findings.
- -
- The main parameter is the minimum mass of magnetic material necessary to achieve maximal separation efficiency. Below this value, parameters such as the pH have a strong impact on the separation efficiency, whereas close to this value the effect becomes smaller.
- -
- Microalgae outer cell walls have many different functional groups, which are mainly negative, but positive groups are also present [54]. In experiments using bacteria cells, and based on zeta-potential results, Li et al. maintained that nano-size effects together with hydrophobic interactions, rather than electrostatic forces, are responsible for the interaction of the bacteria with the oleate-coated iron oxide nanoparticles used [20]. Cerff and colleagues questioned this outcome, adding that the possible existence of locally-distributed positive charges of, e.g., protonated amino groups, may still make the electrostatic interaction responsible for adsorption [32]. Then, positively charged groups may meet negatively charged groups across the whole pH range, due to the complexity of the components present and the various dissociation constants (the so-called Lewis acid-base properties). Therefore, even when repulsion is expected from the zeta potential values (netto charge) of nanoparticles and algae, the patchwork model for charge distribution on the surface makes interaction possible, as recently presented by us [57]. Furthermore, the zeta potential of the particles depends strongly on the environmental conditions [40,57]. Note that the zeta potential measurement, although a fast, easy, and widely used method for gaining surface charge information, is not completely appropriate for microalgae systems due to the complex medium present, as explained by Lim et al. [31].
- -
- On the other hand, if the results of detachment experiments are taken into account, it is difficult to understand why only the addition of deionized water has such a strong impact on detachment, whereas dilution by adding BG-11 medium and strong pH shift to pH 12 have no impact. One possible explanation is that in both cases, enough salt is still present (in the second case due to the counterions for the pH adjustment) and the ions at the interphase lead to chelate complexation and molecular bridging with the complex cell wall (polysaccharides, lipids, proteins) hampering detachment [58]. In contrast, the addition of salt-free water strongly decreases the ion concentration in the double layer and therefore leads to the cancellation of electrostatic and/or ionic effects and coordination bonding. Procházková et al. obtained low detachment values and used the extended Derjaguin–Landau–Verwey–Overbeek theory to explain this result and to analyze the adhesion mechanism, concluding that this theory is not sufficient to describe the observations and, therefore, covalent forces are probably also involved in the interaction [36]. They worked with coated particles, which could open the door to covalent interactions. In the case of bare iron oxides, as presented in this paper, the presence of covalent forces is difficult to imagine, due to the high efficiency of detachment using salt-free water. Nevertheless, osmotic properties of the algae may be the driving force or at least an important factor. This is an essential question which should be investigated in the future.
- -
- -
- Finally, the agglomeration behavior of BIONs and of microalgae is influenced by environmental conditions and also impacts harvesting efficiency. Changes in the agglomeration behavior of the system have not yet been considered in studies published on magnetic separation of microalgae, although they are probably one key for better understanding the system dependencies.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Specht, E.; Miyake-Stoner, S.; Mayfield, S. Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 2010, 32, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Gupta, S.; Cho, W.-M.; Kim, J.; Lee, S.; Jeong, K.-H.; Lee, D.; Choi, H.-C. Microalgae Potential and Multiple Roles—Current Progress and Future Prospects—An Overview. Sustainability 2016, 8, 1215. [Google Scholar] [CrossRef]
- Barry, A.; Wolfe, A.; English, C.; Ruddick, C.; Lambert, D. 2016 National Algal Biofuels Technology Review; DOE U.S. Department of Energy: Washington, DC, USA, 2016.
- Odjadjare, E.C.; Mutanda, T.; Olaniran, A.O. Potential biotechnological application of microalgae: A critical review. Crit. Rev. Biotechnol. 2017, 37, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I. The Evolution and Versatility of Microalgal Biotechnology: A Review. Compr. Rev Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Hariskos, I.; Posten, C. Biorefinery of microalgae—Opportunities and constraints for different production scenarios. Biotechnol. J. 2014, 9, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.P.; Löwe, H.; Schmid, V.; Mundt, S.; Weuster-Botz, D. Model-supported phototrophic growth studies with Scenedesmus obtusiusculus in a flat-plate photobioreactor. Biotechnol. Bioeng. 2017, 114, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.D.; Mota, A.; Teixeira, J.A.; Vicente, A.A. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol. Adv. 2015, 33, 1228–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, C.; Vaidyanathan, S. Microalgae: A robust “green bio-bridge” between energy and environment. Crit. Rev. Biotechnol. 2018, 38, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Tang, M.S.Y.; Nagarajan, D.; Ling, T.C.; Ooi, C.-W.; Chang, J.-S. A Holistic Approach to Managing Microalgae for Biofuel Applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Apel, A.C.; Pfaffinger, C.E.; Basedahl, N.; Mittwollen, N.; Göbel, J.; Sauter, J.; Brück, T.; Weuster-Botz, D. Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res. 2017, 25, 381–390. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Harvesting microalgae by magnetic separation: A review. Algal Res. 2015, 9, 178–185. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.-W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Borlido, L.; Azevedo, A.M.; Roque, A.C.A.; Aires-Barros, M.R. Magnetic separations in biotechnology. Biotechnol. Adv. 2013, 31, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-G.; Gao, H.-S.; Li, W.-L.; Xing, J.-M.; Liu, H.-Z. In situ magnetic separation and immobilization of dibenzothiophene-desulfurizing bacteria. Bioresour. Technol. 2009, 100, 5092–5096. [Google Scholar] [CrossRef] [PubMed]
- Rascol, E.; Daurat, M.; Da Silva, A.; Maynadier, M.; Dorandeu, C.; Charnay, C.; Garcia, M.; Lai-Kee-Him, J.; Bron, P.; Auffan, M.; et al. Biological Fate of Fe3O4 Core-Shell Mesoporous Silica Nanoparticles Depending on Particle Surface Chemistry. Nanomaterials 2017, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Fraga-García, P.; Merck, G.K.; Bodensteiner, F.A.; Heissler, S.; Günther, S.; Berensmeier, S. On the Nature of Interactions of Amino Acids with Bare Magnetite Nanoparticles. J. Phys. Chem. C 2015, 119, 23032–23041. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Blank-Shim, S.A.; Scheifele, I.; Fraga-Garcia, P.; Berensmeier, S. Peptide binding to metal oxide nanoparticles. Faraday Discuss. 2017, 204, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.; Blank-Shim, S.A.; Borkowska-Panek, M.; Anand, P.; Fraga-García, P.; Fink, K.; Wenzel, W.; Berensmeier, S. Experimental characterization and simulation of amino acid and peptide interactions with inorganic materials. Eng. Life Sci. 2018, 18, 84–100. [Google Scholar] [CrossRef]
- Roth, H.-C.; Schwaminger, S.P.; Peng, F.; Berensmeier, S. Immobilization of Cellulase on Magnetic Nanocarriers. ChemistryOpen 2016, 5, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Fraga-García, P.; Selbach, F.; Hein, F.G.; Fuß, E.C.; Surya, R.; Roth, H.-C.; Blank-Shim, S.A.; Wagner, F.E.; Heissler, S.; et al. Bio-nano interactions: Cellulase on iron oxide nanoparticle surfaces. Adsorption 2017, 23, 281–292. [Google Scholar] [CrossRef]
- Fraga García, P.; von Roman, M.F.; Reinlein, S.; Wolf, M.; Berensmeier, S. Impact of Nanoparticle Aggregation on Protein Recovery through a Pentadentate Chelate Ligand on Magnetic Carriers. ACS Appl. Mater. Interfaces 2014, 6, 13607–13616. [Google Scholar] [CrossRef] [PubMed]
- Fraga García, P.; Brammen, M.; Wolf, M.; Reinlein, S.; von Roman, M.F.; Berensmeier, S. High-gradient magnetic separation for technical scale protein recovery using low cost magnetic nanoparticles. Sep. Purif. Technol. 2015, 150, 29–36. [Google Scholar] [CrossRef]
- Roth, H.-C.; Prams, A.; Lutz, M.; Ritscher, J.; Raab, M.; Berensmeier, S. A High-Gradient Magnetic Separator for Highly Viscous Process Liquors in Industrial Biotechnology. Chem. Eng. Technol. 2016, 39, 469–476. [Google Scholar] [CrossRef]
- Bitton, G.; Fox, J.L.; Strickland, H.G. Removal of algae from Florida lakes by magnetic filtration. Appl. Microbiol. 1975, 30, 905–908. [Google Scholar] [PubMed]
- Lim, J.K.; Chieh, D.C.J.; Jalak, S.A.; Toh, P.Y.; Yasin, N.H.M.; Ng, B.W.; Ahmad, A.L. Rapid magnetophoretic separation of microalgae. Small 2012, 8, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Cerff, M.; Morweiser, M.; Dillschneider, R.; Michel, A.; Menzel, K.; Posten, C. Harvesting fresh water and marine algae by magnetic separation: Screening of separation parameters and high gradient magnetic filtration. Bioresour. Technol. 2012, 118, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, F.; Zhang, B. Removal of algal blooms in freshwater using magnetic polymer. Water Sci. Technol. 2009, 59, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Lee, K.; Lee, S.Y.; Jeon, S.G.; Na, J.-G.; Oh, Y.-K.; Park, S.B. Effect of barium ferrite particle size on detachment efficiency in magnetophoretic harvesting of oleaginous Chlorella sp. Bioresour. Technol. 2014, 152, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Procházková, G.; Šafařík, I.; Brányik, T. Surface Modification of Chlorella Vulgaris Cells Using Magnetite Particles. Procedia Eng. 2012, 42, 1778–1787. [Google Scholar] [CrossRef]
- Prochazkova, G.; Podolova, N.; Safarik, I.; Zachleder, V.; Branyik, T. Physicochemical approach to freshwater microalgae harvesting with magnetic particles. Colloids Surf. B 2013, 112, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, C.; Wang, F.; Zheng, S.; Liu, C.-Z. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour. Technol. 2011, 102, 10047–10051. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lee, K.; Oh, Y.-K. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: A review. Bioresour. Technol. 2015, 184, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Safarik, I.; Pospiskova, K.; Baldikova, E. Magnetic Particles for Microalgae Separation and Biotechnology. In Food Bioactives; Puri, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 153–169. [Google Scholar]
- Prochazkova, G.; Safarik, I.; Branyik, T. Harvesting microalgae with microwave synthesized magnetic microparticles. Bioresour. Technol. 2013, 130, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Toh, P.Y.; Ng, B.W.; Ahmad, A.L.; Chieh, D.C.J.; Lim, J. The role of particle-to-cell interactions in dictating nanoparticle aided magnetophoretic separation of microalgal cells. Nanoscale 2014, 6, 12838–12848. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.-C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: A model based study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- Waterbury, J.B.; Stanier, R.Y. Isolation and Growth of Cyanobacteria from Marine and Hypersaline Environments. In The Prokaryotes; Starr, M.P., Stolp, H., Trüper, H.G., Balows, A., Schlegel, H.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 221–223. [Google Scholar]
- Koller, A.P.; Wolf, L.; Weuster-Botz, D. Reaction engineering analysis of Scenedesmus ovalternus in a flat-plate gas-lift photobioreactor. Bioresour. Technol. 2017, 225, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Heidenreich, E.; Franzreb, M.; Frankenfeld, K. Purification of equine chorionic gonadotropin (eCG) using magnetic ion exchange adsorbents in combination with high-gradient magnetic separation. Biotechnol. Prog. 2015, 31, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef]
- Franzreb, M.; Siemann-Herzberg, M.; Hobley, T.J.; Thomas, O.R.T. Protein purification using magnetic adsorbent particles. Appl. Microbiol. Biotechnol. 2006, 70, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Paul, A.; Gangopadhyay, M.; Singh, N.D.P.; Sen, R. Smart and Reusable Biopolymer Nanocomposite for Simultaneous Microalgal Biomass Harvesting and Disruption: Integrated Downstream Processing for a Sustainable Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 852–861. [Google Scholar] [CrossRef]
- Ge, S.; Agbakpe, M.; Wu, Z.; Kuang, L.; Zhang, W.; Wang, X. Influences of surface coating, UV irradiation and magnetic field on the algae removal using magnetite nanoparticles. Environ. Sci. Technol. 2015, 49, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-R.; Guo, C.; Xu, L.; Wang, F.; Wang, S.-K.; Hu, Z.; Liu, C.-Z. A magnetic separator for efficient microalgae harvesting. Bioresour. Technol. 2014, 158, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Wang, F.; Hu, Y.-R.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Magnetic flocculant for high efficiency harvesting of microalgal cells. ACS Appl. Mater. Interfaces 2014, 6, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-D.; Hiltunen, E.; Li, Z. Using magnetic materials to harvest microalgal biomass: Evaluation of harvesting and detachment efficiency. Environ. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Y.; Tao, Y.; Zhang, Y.; Li, A.; Li, T.; Sang, M.; Zhang, C. Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol. Biofuels 2013, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, H.; Song, S. Cell surface characterization of some oleaginous green algae. J. Appl. Phycol. 2016, 28, 2323–2332. [Google Scholar] [CrossRef]
- Hu, Y.-R.; Wang, F.; Wang, S.-K.; Liu, C.-Z.; Guo, C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.Y.; Praveenkumar, R.; Kim, B.; Seo, J.Y.; Jeon, S.G.; Na, J.-G.; Park, J.-Y.; Kim, D.-M.; Oh, Y.-K. Repeated use of stable magnetic flocculant for efficient harvest of oleaginous Chlorella sp. Bioresour. Technol. 2014, 167, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Blank-Shim, S.A.; Schwaminger, S.P.; Borkowska-Panek, M.; Anand, P.; Yamin, P.; Fraga-García, P.; Fink, K.; Wenzel, W.; Berensmeier, S. Binding patterns of homo-peptides on bare magnetic nanoparticles: Insights into environmental dependence. Sci. Rep. 2017, 7, 14047. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef] [PubMed]
- Toh, P.Y.; Yeap, S.P.; Kong, L.P.; Ng, B.W.; Chan, D.J.C.; Ahmad, A.L.; Lim, J.K. Magnetophoretic removal of microalgae from fishpond water: Feasibility of high gradient and low gradient magnetic separation. Chem. Eng. J. 2012, 211–212, 22–30. [Google Scholar] [CrossRef]
- Ebeler, M.; Pilgram, F.; Wolz, K.; Grim, G.; Franzreb, M. Magnetic Separation on a New Level: Characterization and Performance Prediction of a cGMP Compliant “Rotor-Stator” High-Gradient Magnetic Separator. Biotechnol. J. 2018, 13, 1700448. [Google Scholar] [CrossRef] [PubMed]
- Karemore, A.; Sen, R. Downstream processing of microalgal feedstock for lipid and carbohydrate in a biorefinery concept: A holistic approach for biofuel applications. RSC Adv. 2016, 6, 29486–29496. [Google Scholar] [CrossRef]
- Eroglu, E.; Raston, C.L. Nanomaterial processing strategies in functional hybrid materials for wastewater treatment using algal biomass. J. Chem. Technol. Biotechnol. 2017, 92, 1862–1867. [Google Scholar] [CrossRef]
- Lv, J.; Feng, J.; Liu, Q.; Xie, S. Microalgal Cultivation in Secondary Effluent: Recent Developments and Future Work. Int. J. Mol. Sci. 2017, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.O.; Deamici, K.M.; Menestrino, B.C.; Garda-Buffon, J.; Costa, J.A.V. Magnetic treatment of microalgae for enhanced product formation. World J. Microbiol. Biotechnol. 2017, 33, 169. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Depraetere, O.; Vandamme, D.; Muylaert, K. Cultivation of Chlorella vulgaris and Arthrospira platensis with recovered phosphorus from wastewater by means of zeolite sorption. Int. J. Mol. Sci. 2015, 16, 4250–4264. [Google Scholar] [CrossRef] [PubMed]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Varma, V.; Yang, S.; Ghasemi, Y.; Berenjian, A. Synthesis and Application of Amine Functionalized Iron Oxide Nanoparticles on Menaquinone-7 Fermentation: A Step towards Process Intensification. Nanomaterials 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]





| Process Step | Fraction | Mass, g | Massalgae, g | Masssalts, g | MassBIONs, g |
|---|---|---|---|---|---|
| Adsorption | Initial | 12.90 | 2.99 | 8.42 | 1.49 |
| Separation | 1 | 6.48 | 0 | 8.6 | 0 |
| Wash Salts recovery | 2 | 2 | |||
| 3 | 0.12 | ||||
| Desorption Algae recovery | 4 | 0.83 | 2.55 | 0 | 0 |
| 5 | 0.45 | ||||
| 6 | 0.74 | ||||
| 7 | 0.25 | ||||
| 8 | 0.15 | ||||
| 9 | 0.13 | ||||
| Recycling BIONs recovery | 10 | 1.45 | x | 0 | 2.05 − x |
| 11 | 0.16 | ||||
| 12 | 0.44 | ||||
| Final | 13.2 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga-García, P.; Kubbutat, P.; Brammen, M.; Schwaminger, S.; Berensmeier, S. Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization. Nanomaterials 2018, 8, 292. https://doi.org/10.3390/nano8050292
Fraga-García P, Kubbutat P, Brammen M, Schwaminger S, Berensmeier S. Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization. Nanomaterials. 2018; 8(5):292. https://doi.org/10.3390/nano8050292
Chicago/Turabian StyleFraga-García, Paula, Peter Kubbutat, Markus Brammen, Sebastian Schwaminger, and Sonja Berensmeier. 2018. "Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization" Nanomaterials 8, no. 5: 292. https://doi.org/10.3390/nano8050292