Endocannabinoid System in the Airways
Abstract
:1. Cannabinoids and the Endocannabinoid System
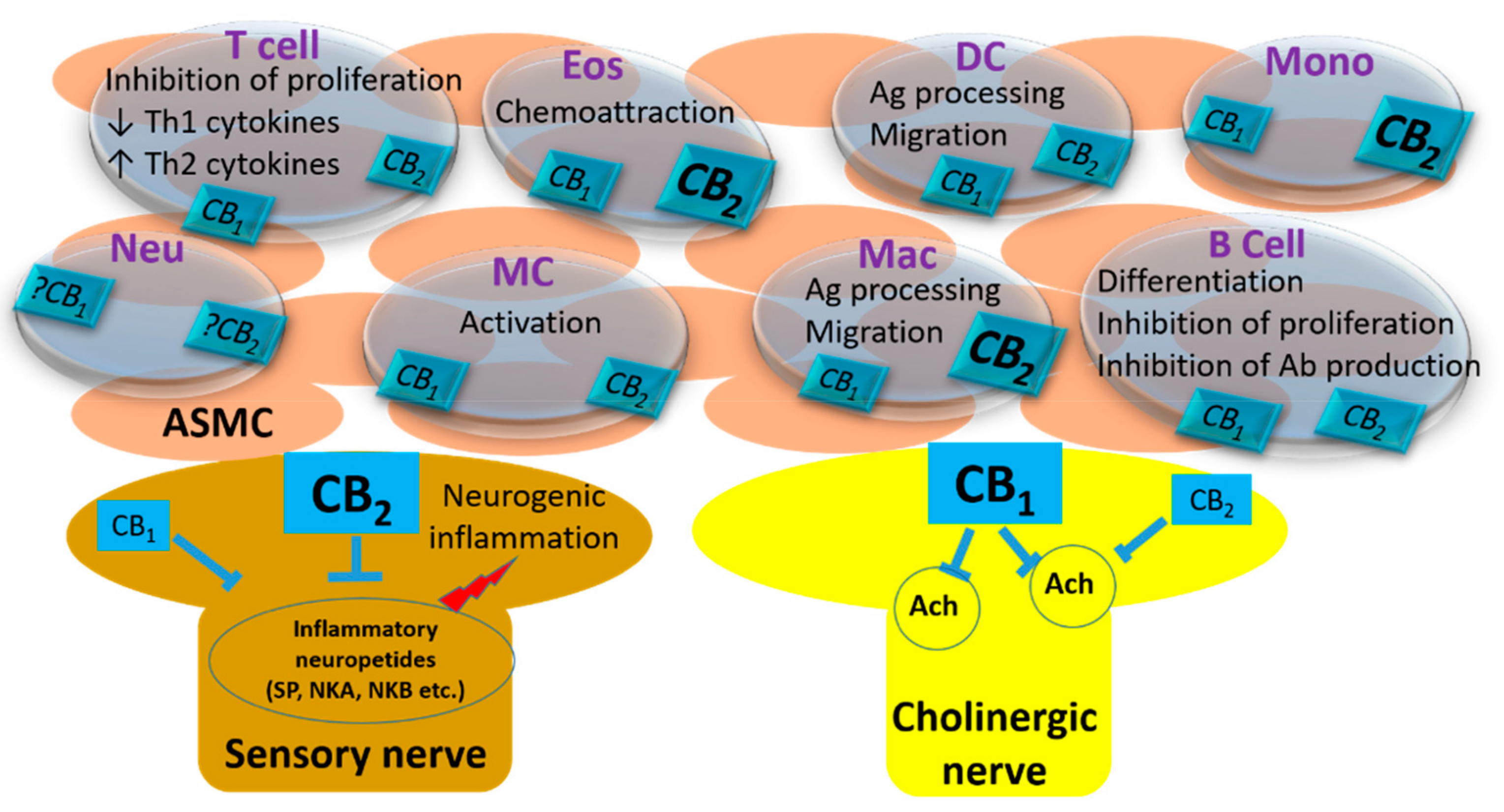
2. The Role of Endocannabinoid System in the Airways
2.1. Airway Reactivity
2.2. Airway Diseases
3. Targeting the Endocannabinoid System for the Treatment of Airway Diseases
4. Conclusions
Funding
Conflicts of Interest
References
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science (NY) 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharm. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Izzo, A.A.; Camilleri, M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut 2008, 57, 1140–1155. [Google Scholar] [CrossRef]
- Di Marzo, V.; Fontana, A. Anandamide, an endogenous cannabinomimetic eicosanoid: ’killing two birds with one stone’. Prostaglandinsleukot. Essent. Fat. Acids. 1995, 53, 1–11. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug. Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharm. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Sun, Y.X.; Tsuboi, K.; Zhao, L.Y.; Okamoto, Y.; Lambert, D.M.; Ueda, N. Involvement of N-acylethanolamine-hydrolyzing acid amidase in the degradation of anandamide and other N-acylethanolamines in macrophages. Biochim. Biophys. Acta 2005, 1736, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef] [Green Version]
- Szafran, B.N.; Lee, J.H.; Borazjani, A.; Morrison, P.; Zimmerman, G.; Andrzejewski, K.L.; Ross, M.K.; Kaplan, B.L.F. Characterization of Endocannabinoid-Metabolizing Enzymes in Human Peripheral Blood Mononuclear Cells under Inflammatory Conditions. Molecules 2018, 23, 3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Borazjani, A.; Matthews, A.T.; Mangum, L.C.; Edelmann, M.J.; Ross, M.K. Identification of palmitoyl protein thioesterase 1 in human THP1 monocytes and macrophages and characterization of unique biochemical activities for this enzyme. Biochemistry 2013, 52, 7559–7574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuo, W.; Leleu-Chavain, N.; Spencer, J.; Sansook, S.; Millet, R.; Chavatte, P. Therapeutic Potential of Fatty Acid Amide Hydrolase, Monoacylglycerol Lipase, and N-Acylethanolamine Acid Amidase Inhibitors. J. Med. Chem. 2017, 60, 4–46. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Sasso, O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111, 5899–5921. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A., 3rd; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharm. 1988, 34, 605–613. [Google Scholar]
- De Petrocellis, L.; Nabissi, M.; Santoni, G.; Ligresti, A. Actions and Regulation of Ionotropic Cannabinoid Receptors. Adv. Pharm. 2017, 80, 249–289. [Google Scholar]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, E.; Larsson, N.; Sjogren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharm. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Delta(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharm. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Huang, B.X.; Kwon, H.; Rashid, M.A.; Kharebava, G.; Desai, A.; Patnaik, S.; Marugan, J.; Kim, H.Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 2016, 7, 13123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.J. Novel cannabinoid receptors. Br. J. Pharm. 2007, 152, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Onaivi, E.S.; Chaudhuri, G. Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. Dna. Seq. 1995, 5, 385–388. [Google Scholar] [CrossRef]
- Shire, D.; Calandra, B.; Rinaldi-Carmona, M.; Oustric, D.; Pessegue, B.; Bonnin-Cabanne, O.; Le Fur, G.; Caput, D.; Ferrara, P. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim. Biophys. Acta 1996, 1307, 132–136. [Google Scholar] [CrossRef]
- Demuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Howlett, A.C. Cannabinoid receptor signaling. Handb. Exp. Pharm. 2005, 168, 53–79. [Google Scholar]
- Rodriguez de Fonseca, F.; Del Arco, I.; Bermudez-Silva, F.J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol. Alcohol. (Oxf. Oxfs.) 2005, 40, 2–14. [Google Scholar] [CrossRef]
- Bouaboula, M.; Rinaldi, M.; Carayon, P.; Carillon, C.; Delpech, B.; Shire, D.; Le Fur, G.; Casellas, P. Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 1993, 214, 173–180. [Google Scholar] [CrossRef]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins. Other. Lipid. Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. FUNCTIONAL EXPRESSION OF BRAIN NEURONAL CB2 CANNABINOID RECEPTORS ARE INVOLVED IN THE EFFECTS OF DRUGS OF ABUSE AND IN DEPRESSION. Ann. NY Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [Green Version]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharm. Toxicol. 2019, 60, 32.1–32.23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdyshev, E.V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids 2000, 108, 169–190. [Google Scholar] [CrossRef]
- D’Argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef]
- Kurabayashi, M.; Takeyoshi, I.; Yoshinari, D.; Matsumoto, K.; Maruyama, I.; Morishita, Y. 2-Arachidonoylglycerol increases in ischemia-reperfusion injury of the rat liver. J. Invest. Surg. 2005, 18, 25–31. [Google Scholar] [CrossRef]
- Muthian, S.; Rademacher, D.J.; Roelke, C.T.; Gross, G.J.; Hillard, C.J. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience 2004, 129, 743–750. [Google Scholar] [CrossRef]
- Panikashvili, D.; Simeonidou, C.; Ben-Shabat, S.; Hanus, L.; Breuer, A.; Mechoulam, R.; Shohami, E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 2001, 413, 527–531. [Google Scholar] [CrossRef]
- Sugamura, K.; Sugiyama, S.; Nozaki, T.; Matsuzawa, Y.; Izumiya, Y.; Miyata, K.; Nakayama, M.; Kaikita, K.; Obata, T.; Takeya, M.; et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009, 119, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, V.L.; Hegde, S.; Cravatt, B.F.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol. Pharm. 2008, 74, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.; Boichot, E.; Corbel, M.; Germain, N.; Lagente, V. Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 1998, 63, PL125–PL129. [Google Scholar] [CrossRef]
- Comelli, F.; Giagnoni, G.; Bettoni, I.; Colleoni, M.; Costa, B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br. J. Pharm. 2007, 152, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csoka, B.; Nemeth, Z.H.; Mukhopadhyay, P.; Spolarics, Z.; Rajesh, M.; Federici, S.; Deitch, E.A.; Batkai, S.; Pacher, P.; Hasko, G. CB2 cannabinoid receptors contribute to bacterial invasion and mortality in polymicrobial sepsis. PLoS ONE 2009, 4, e6409. [Google Scholar] [CrossRef] [Green Version]
- Engel, M.A.; Kellermann, C.A.; Burnat, G.; Hahn, E.G.; Rau, T.; Konturek, P.C. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J. Physiol. Pharm. 2010, 61, 89–97. [Google Scholar]
- Kaminski, N.E.; Koh, W.S.; Yang, K.H.; Lee, M.; Kessler, F.K. Suppression of the humoral immune response by cannabinoids is partially mediated through inhibition of adenylate cyclase by a pertussis toxin-sensitive G-protein coupled mechanism. Biochem. Pharm. 1994, 48, 1899–1908. [Google Scholar] [CrossRef]
- Titishov, N.; Mechoulam, R.; Zimmerman, A.M. Stereospecific effects of (-)- and (+)-7-hydroxy-delta-6-tetrahydrocannabinol-dimethylheptyl on the immune system of mice. Pharmacology 1989, 39, 337–349. [Google Scholar] [CrossRef]
- Lu, T.; Newton, C.; Perkins, I.; Friedman, H.; Klein, T.W. Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila. Eur. J. Pharmacol. 2006, 532, 170–177. [Google Scholar] [CrossRef]
- Klein, T.W.; Lane, B.; Newton, C.A.; Friedman, H. The cannabinoid system and cytokine network. Proc. Soc. Exp. Biol. Med. 2000, 225, 1–8. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.A.; Nakachi, N.; Friedman, H. Delta 9-tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12, and IL-12 receptor beta 2 responses to Legionella pneumophila infection. J. Immunol. (Baltimore, Md. 1950) 2000, 164, 6461–6466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. (Baltimore, Md. 1950) 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishkin, E.M.; Cabral, G.A. delta-9-Tetrahydrocannabinol decreases host resistance to herpes simplex virus type 2 vaginal infection in the B6C3F1 mouse. J. Gen. Virol. 1985, 66, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Carayon, P.; Marchand, J.; Dussossoy, D.; Derocq, J.M.; Jbilo, O.; Bord, A.; Bouaboula, M.; Galiegue, S.; Mondiere, P.; Penarier, G.; et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood 1998, 92, 3605–3615. [Google Scholar] [CrossRef]
- Sacerdote, P.; Massi, P.; Panerai, A.E.; Parolaro, D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 2000, 109, 155–163. [Google Scholar] [CrossRef]
- McCoy, K.L.; Matveyeva, M.; Carlisle, S.J.; Cabral, G.A. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: Evidence for CB2 receptor participation. J. Pharm. Exp. 1999, 289, 1620–1625. [Google Scholar]
- Vannacci, A.; Giannini, L.; Passani, M.B.; Di Felice, A.; Pierpaoli, S.; Zagli, G.; Fantappie, O.; Mazzanti, R.; Masini, E.; Mannaioni, P.F. The endocannabinoid 2-arachidonylglycerol decreases the immunological activation of Guinea pig mast cells: Involvement of nitric oxide and eicosanoids. J. Pharm. Exp. 2004, 311, 256–264. [Google Scholar] [CrossRef]
- Samson, M.T.; Small-Howard, A.; Shimoda, L.M.; Koblan-Huberson, M.; Stokes, A.J.; Turner, H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J. Immunol. (Baltimore, Md. 1950) 2003, 170, 4953–4962. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Biro, T.; Tsuruta, D.; Toth, B.I.; Kromminga, A.; Zakany, N.; Zimmer, A.; Funk, W.; Gibbs, B.F.; Zimmer, A.; et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J. Allergy Clin. Immunol. 2012, 129, 726–738.e8. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Zakany, N.; Hundt, T.; Emelianov, V.; Tsuruta, D.; Schafer, C.; Kloepper, J.E.; Biro, T.; Paus, R. Cannabinoid receptor 1 controls human mucosal-type mast cell degranulation and maturation in situ. J. Allergy Clin. Immunol. 2013, 132, 182–193. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharm. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Alexander, S.P.; Kendall, D.A.; Bennett, A.J. Cannabinoids and PPARalpha signalling. Biochem. Soc. Trans. 2006, 34, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.P.; Sutter, M.E.; Albertson, T.E. Marijuana: Respiratory tract effects. Clin. Rev. Allergy Immunol. 2014, 46, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Gong, H., Jr.; Fligiel, S.; Tashkin, D.P.; Barbers, R.G. Tracheobronchial changes in habitual, heavy smokers of marijuana with and without tobacco. Am. Rev. Respir. Dis. 1987, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Fligiel, S.E.; Beals, T.F.; Tashkin, D.P.; Paule, M.G.; Scallet, A.C.; Ali, S.F.; Bailey, J.R.; Slikker, W., Jr. Marijuana exposure and pulmonary alterations in primates. Pharm. Biochem. Behav. 1991, 40, 637–642. [Google Scholar] [CrossRef]
- Giannini, L.; Nistri, S.; Mastroianni, R.; Cinci, L.; Vannacci, A.; Mariottini, C.; Passani, M.B.; Mannaioni, P.F.; Bani, D.; Masini, E. Activation of cannabinoid receptors prevents antigen-induced asthma-like reaction in guinea pigs. J. Cell Mol. Med. 2008, 12, 2381–2394. [Google Scholar] [CrossRef] [Green Version]
- Zoerner, A.A.; Stichtenoth, D.O.; Engeli, S.; Batkai, S.; Winkler, C.; Schaumann, F.; Janke, J.; Holz, O.; Krug, N.; Tsikas, D.; et al. Allergen challenge increases anandamide in bronchoalveolar fluid of patients with allergic asthma. Clin. Pharm. 2011, 90, 388–391. [Google Scholar] [CrossRef]
- Oka, S.; Ikeda, S.; Kishimoto, S.; Gokoh, M.; Yanagimoto, S.; Waku, K.; Sugiura, T. 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J. Leukoc Biol. 2004, 76, 1002–1009. [Google Scholar] [CrossRef] [Green Version]
- Chouinard, F.; Lefebvre, J.S.; Navarro, P.; Bouchard, L.; Ferland, C.; Lalancette-Hebert, M.; Marsolais, D.; Laviolette, M.; Flamand, N. The endocannabinoid 2-arachidonoyl-glycerol activates human neutrophils: Critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J. Immunol. (Baltimore, Md. 1950) 2011, 186, 3188–3196. [Google Scholar] [CrossRef] [Green Version]
- Chouinard, F.; Turcotte, C.; Guan, X.; Larose, M.C.; Poirier, S.; Bouchard, L.; Provost, V.; Flamand, L.; Grandvaux, N.; Flamand, N. 2-Arachidonoyl-glycerol- and arachidonic acid-stimulated neutrophils release antimicrobial effectors against E. coli, S. aureus, HSV-1, and RSV. J. Leukoc Biol. 2013, 93, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montecucco, F.; Burger, F.; Mach, F.; Steffens, S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1145–H1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, J.T.; Harui, A.; Kiertscher, S.M.; Roth, J.D.; Roth, M.D. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J. Neuroimmune Pharm. 2013, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. Impact of Cannabis, Cannabinoids, and Endocannabinoids in the Lungs. Front. Pharm. 2016, 7, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, S.; Oka, S.; Gokoh, M.; Sugiura, T. Chemotaxis of human peripheral blood eosinophils to 2-arachidonoylglycerol: Comparison with other eosinophil chemoattractants. Int. Arch. Allergy Immunol. 2006, 140, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Larose, M.C.; Turcotte, C.; Chouinard, F.; Ferland, C.; Martin, C.; Provost, V.; Laviolette, M.; Flamand, N. Mechanisms of human eosinophil migration induced by the combination of IL-5 and the endocannabinoid 2-arachidonoyl-glycerol. J. Allergy Clin. Immunol. 2014, 133, 1480–1482. [Google Scholar] [CrossRef]
- Frei, R.B.; Luschnig, P.; Parzmair, G.P.; Peinhaupt, M.; Schranz, S.; Fauland, A.; Wheelock, C.E.; Heinemann, A.; Sturm, E.M. Cannabinoid receptor 2 augments eosinophil responsiveness and aggravates allergen-induced pulmonary inflammation in mice. Allergy 2016, 71, 944–956. [Google Scholar] [CrossRef]
- Matias, I.; Pochard, P.; Orlando, P.; Salzet, M.; Pestel, J.; Di Marzo, V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur. J. Biochem. 2002, 269, 3771–3778. [Google Scholar] [CrossRef] [Green Version]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. (Baltimore, Md. 1950) 2004, 173, 2373–2382. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, R.; Tohyama, Y.; Matsusaka, S.; Naruse, H.; Kinoshita, E.; Tsujioka, T.; Katsumata, Y.; Yamamura, H. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J. Biol. Chem. 2006, 281, 12908–12918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashkin, D.P.; Shapiro, B.J.; Frank, I.M. Acute pulmonary physiologic effects of smoked marijuana and oral (Delta)9 -tetrahydrocannabinol in healthy young men. N. Engl. J. Med. 1973, 289, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Vachon, L.; FitzGerald, M.X.; Solliday, N.H.; Gould, I.A.; Gaensler, E.A. Single-dose effects of marihuana smoke. Bronchial dynamics and respiratory-center sensitivity in normal subjects. N. Engl. J. Med. 1973, 288, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Shapiro, B.J.; Frank, I.M. Acute effects of smoked marijuana and oral delta9-tetrahydrocannabinol on specific airway conductance in asthmatic subjects. Am. Rev. Respir. Dis. 1974, 109, 420–428. [Google Scholar]
- Tashkin, D.P.; Shapiro, B.J.; Lee, Y.E.; Harper, C.E. Effects of smoked marijuana in experimentally induced asthma. Am. Rev. Respir. Dis. 1975, 112, 377–386. [Google Scholar]
- Hartley, J.P.; Nogrady, S.G.; Seaton, A. Bronchodilator effect of delta1-tetrahydrocannabinol. Br. J. Clin. Pharm. 1978, 5, 523–525. [Google Scholar] [CrossRef] [Green Version]
- Abboud, R.T.; Sanders, H.D. Effect of oral administration of delta-tetrahydrocannabinol on airway mechanics in normal and asthmatic subjects. Chest 1976, 70, 480–485. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Reiss, S.; Shapiro, B.J.; Calvarese, B.; Olsen, J.L.; Lodge, J.W. Bronchial effects of aerosolized delta 9-tetrahydrocannabinol in healthy and asthmatic subjects. Am. Rev. Respir. Dis. 1977, 115, 57–65. [Google Scholar]
- Calignano, A.; Katona, I.; Desarnaud, F.; Giuffrida, A.; La Rana, G.; Mackie, K.; Freund, T.F.; Piomelli, D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature 2000, 408, 96–101. [Google Scholar] [CrossRef]
- Yousif, M.H.; Oriowo, M.A. Inhibitory effects of cannabinoid receptor ligands on electrically-evoked responses in rat isolated tracheal ring segments. Pharm. Res. 1999, 40, 415–421. [Google Scholar] [CrossRef]
- Spicuzza, L.; Haddad, E.-B.; Birrell, M.; Ling, A.; Clarke, D.; Venkatesan, P.; Barnes, P.J.; Belvisi, M.G. Characterization of the effects of cannabinoids on guinea-pig tracheal smooth muscle tone: Role in the modulation of acetylcholine release from parasympathetic nerves. Br. J. Pharm. 2000, 130, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Naline, E.; Buenestado, A.; Faisy, C.; Alvarez, J.C.; Salvator, H.; Abrial, C.; Advenier, C.; Zemoura, L.; Devillier, P. Cannabinoids inhibit cholinergic contraction in human airways through prejunctional CB1 receptors. Br. J. Pharm. 2014, 171, 2767–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozkurt, T.E.; Larsson, O.; Adner, M. Stimulation of cannabinoid CB1 receptors prevents nerve-mediated airway hyperreactivity in NGF-induced inflammation in mouse airways. Eur. J. Pharm. 2016, 776, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Batkai, S.; Rajesh, M.; Mukhopadhyay, P.; Hasko, G.; Liaudet, L.; Cravatt, B.F.; Csiszar, A.; Ungvari, Z.; Pacher, P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H909–H918. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horvath, B.; Rajesh, M.; Matsumoto, S.; Saito, K.; Batkai, S.; Patel, V.; Tanchian, G.; Gao, R.Y.; Cravatt, B.F.; et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic. Biol. Med. 2011, 50, 179–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Yao, H.; Cheng, Y.; Gong, D.; Liao, X.; Wang, R. Effects of monoacylglycerol lipase inhibitor URB602 on lung ischemia-reperfusion injury in mice. Biochem. Biophys. Res. Commun. 2018, 506, 578–584. [Google Scholar] [CrossRef]
- Yin, H.; Li, X.; Xia, R.; Yi, M.; Cheng, Y.; Wu, Y.; Ke, B.; Wang, R. Posttreatment with the Fatty Acid Amide Hydrolase Inhibitor URB937 Ameliorates One-Lung Ventilation-Induced Lung Injury in a Rabbit Model. J. Surg. Res. 2019, 239, 83–91. [Google Scholar] [CrossRef]
- Roviezzo, F.; Rossi, A.; Caiazzo, E.; Orlando, P.; Riemma, M.A.; Iacono, V.M.; Guarino, A.; Ialenti, A.; Cicala, C.; Peritore, A.; et al. Palmitoylethanolamide Supplementation during Sensitization Prevents Airway Allergic Symptoms in the Mouse. Front. Pharm. 2017, 8, 857. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharm. 2002, 42, 11S–19S. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dudasova, A.; Keir, S.D.; Parsons, M.E.; Molleman, A.; Page, C.P. The effects of cannabidiol on the antigen-induced contraction of airways smooth muscle in the guinea-pig. Pulm. Pharm. 2013, 26, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardo, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fontecha, M.; Eiwegger, T.; Jartti, T.; Rueda-Zubiaurre, A.; Tiringer, K.; Stepanow, J.; Puhakka, T.; Ruckert, B.; Ortega-Gutierrez, S.; Lopez-Rodriguez, M.L.; et al. The expression of cannabinoid receptor 1 is significantly increased in atopic patients. J. Allergy Clin. Immunol. 2014, 133, 926–929.e2. [Google Scholar] [CrossRef]
- Tahamtan, A.; Tavakoli-Yaraki, M.; Shadab, A.; Rezaei, F.; Marashi, S.M.; Shokri, F.; Mokhatri-Azad, T.; Salimi, V. The Role of Cannabinoid Receptor 1 in the Immunopathology of Respiratory Syncytial Virus. Viral. Immunol. 2018, 31, 292–298. [Google Scholar] [CrossRef]
- Tahamtan, A.; Samieipoor, Y.; Nayeri, F.S.; Rahbarimanesh, A.A.; Izadi, A.; Rashidi-Nezhad, A.; Tavakoli-Yaraki, M.; Farahmand, M.; Bont, L.; Shokri, F.; et al. Effects of cannabinoid receptor type 2 in respiratory syncytial virus infection in human subjects and mice. Virulence 2018, 9, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Abohalaka, R.; Bozkurt, T.E.; Onder, S.C.; Sahin-Erdemli, I. The effects of fatty acid amide hydrolase and monoacylglycerol lipase inhibitors in lipopolysaccharide-induced airway inflammation and tracheal hyperreactivity in mice. Eur. Respir. J. 2018, 52, PA5249. [Google Scholar]
- Belvisi, M.G.; Patel, H.J.; Freund-Michel, V.; Hele, D.J.; Crispino, N.; Birrell, M.A. Inhibitory activity of the novel CB2 receptor agonist, GW833972A, on guinea-pig and human sensory nerve function in the airways. Br. J. Pharm. 2008, 155, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, T.E.; Kaya, Y.; Durlu-Kandilci, N.T.; Onder, S.; Sahin-Erdemli, I. The effect of cannabinoids on dinitrofluorobenzene-induced experimental asthma in mice. Respir. Physiol. Neurobiol. 2016, 231, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Engel, T.; Aguilar-Pimentel, J.A.; Zimmer, A.; Jakob, T.; Behrendt, H.; Mempel, M. Beneficial effects of cannabinoids (CB) in a murine model of allergen-induced airway inflammation: Role of CB1/CB2 receptors. Immunobiology 2011, 216, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Y.; D’Agostino, B.; Risse, P.A.; Marrocco, G.; Naline, E.; Zhang, Y.; Chen, H.Z.; Finance, O.; Rinaldi-Carmona, M.; Rossi, F.; et al. Cannabinoid CB(2) receptor activation prevents bronchoconstriction and airway oedema in a model of gastro-oesophageal reflux. Eur. J. Pharm. 2007, 573, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Stengel, P.W.; Cockerham, S.L.; Silbaugh, S.A. Inhaled anandamide reduces leukotriene D4-induced airway obstruction in guinea pigs. Eur. J. Pharm. 2007, 557, 66–68. [Google Scholar] [CrossRef]
- Gkoumassi, E.; Dekkers, B.G.J.; Dröge, M.J.; Elzinga, C.R.S.; Schmidt, M.; Meurs, H.; Zaagsma, J.; Nelemans, S.A. Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells. Br. J. Pharm. 2007, 151, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Jan, T.-R.; Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol. Appl. Pharm. 2003, 188, 24–35. [Google Scholar] [CrossRef]
- Patel, H.J.; Birrell, M.A.; Crispino, N.; Hele, D.J.; Venkatesan, P.; Barnes, P.J.; Yacoub, M.H.; Belvisi, M.G. Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br. J. Pharm. 2003, 140, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Ferrini, M.E.; Hong, S.; Stierle, A.; Stierle, D.; Stella, N.; Roberts, K.; Jaffar, Z. CB2 receptors regulate natural killer cells that limit allergic airway inflammation in a murine model of asthma. Allergy 2017, 72, 937–947. [Google Scholar] [CrossRef]
- Shang, V.C.; O’Sullivan, S.E.; Kendall, D.A.; Roberts, R.E. The endogenous cannabinoid anandamide increases human airway epithelial cell permeability through an arachidonic acid metabolite. Pharm. Res. 2016, 105, 152–163. [Google Scholar] [CrossRef]
- Wortley, M.A.; Adcock, J.J.; Dubuis, E.D.; Maher, S.A.; Bonvini, S.J.; Delescluse, I.; Kinloch, R.; McMurray, G.; Perros-Huguet, C.; Papakosta, M.; et al. Targeting fatty acid amide hydrolase as a therapeutic strategy for antitussive therapy. Eur. Respir. J. 2017, 50, 1700782. [Google Scholar] [CrossRef] [Green Version]
- Costola-de-Souza, C.; Ribeiro, A.; Ferraz-de-Paula, V.; Calefi, A.S.; Aloia, T.P.; Gimenes-Junior, J.A.; de Almeida, V.I.; Pinheiro, M.L.; Palermo-Neto, J. Monoacylglycerol lipase (MAGL) inhibition attenuates acute lung injury in mice. PLoS ONE 2013, 8, e77706. [Google Scholar] [CrossRef] [PubMed]
- Cinar, R.; Iyer, M.R.; Liu, Z.; Cao, Z.; Jourdan, T.; Erdelyi, K.; Godlewski, G.; Szanda, G.; Liu, J.; Park, J.K.; et al. Hybrid inhibitor of peripheral cannabinoid-1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI Insight 2016, 1, e87336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patsenker, E.; Stoll, M.; Millonig, G.; Agaimy, A.; Wissniowski, T.; Schneider, V.; Mueller, S.; Brenneisen, R.; Seitz, H.K.; Ocker, M.; et al. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol. Med. 2011, 17, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Racz, I.; Siegmund, S.V.; Cara, E.; Granzow, M.; Schierwagen, R.; Klein, S.; Wojtalla, A.; Hennenberg, M.; Huss, S.; et al. Role of cannabinoid receptors in alcoholic hepatic injury: Steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver. Int. 2011, 31, 860–870. [Google Scholar] [CrossRef]
- Lecru, L.; Desterke, C.; Grassin-Delyle, S.; Chatziantoniou, C.; Vandermeersch, S.; Devocelle, A.; Vernochet, A.; Ivanovski, N.; Ledent, C.; Ferlicot, S.; et al. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int. 2015, 88, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.L.; Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Wang, J.Y.; Huang, Y.T.; Wang, S.Y.; Wang, F.S. Cannabinoid receptor 1 disturbance of PPARgamma2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J. Mol. Med. (Berl.) 2014, 92, 779–792. [Google Scholar] [CrossRef]
- Slavic, S.; Lauer, D.; Sommerfeld, M.; Kemnitz, U.R.; Grzesiak, A.; Trappiel, M.; Thone-Reineke, C.; Baulmann, J.; Paulis, L.; Kappert, K.; et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J. Mol. Med. (Berl.) 2013, 91, 811–823. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Natale, M.; Gianchecchi, E.; Capecchi, P.L.; Montilli, C.; Zimbone, S.; Castrichini, M.; Balistreri, E.; Ricci, G.; Selvi, E.; et al. Adenosine A2A receptor activation stimulates collagen production in sclerodermic dermal fibroblasts either directly and through a cross-talk with the cannabinoid system. J. Mol. Med. (Berl.) 2012, 90, 331–342. [Google Scholar] [CrossRef]
- Bronova, I.; Smith, B.; Aydogan, B.; Weichselbaum, R.R.; Vemuri, K.; Erdelyi, K.; Makriyannis, A.; Pacher, P.; Berdyshev, E.V. Protection from Radiation-Induced Pulmonary Fibrosis by Peripheral Targeting of Cannabinoid Receptor-1. Am. J. Respir. Cell Mol. Biol. 2015, 53, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Cinar, R.; Gochuico, B.R.; Iyer, M.R.; Jourdan, T.; Yokoyama, T.; Park, J.K.; Coffey, N.J.; Pri-Chen, H.; Szanda, G.; Liu, Z.; et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight 2017, 2, e92281. [Google Scholar] [CrossRef]
- Valdeolivas, S.; Pazos, M.R.; Bisogno, T.; Piscitelli, F.; Iannotti, F.A.; Allara, M.; Sagredo, O.; Di Marzo, V.; Fernandez-Ruiz, J. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: A potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis. 2013, 4, e862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.; Ni, J.; Ling, K.H.; Acheampong, A.; Tang-Liu, D.D.; Burk, R.; Cravatt, B.F.; Woodward, D. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J. Lipid. Res. 2004, 45, 757–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggins, J.P.; Smart, T.S.; Langman, S.; Taylor, L.; Young, T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012, 153, 1837–1846. [Google Scholar] [PubMed]
- Kerbrat, A.; Ferre, J.C.; Fillatre, P.; Ronziere, T.; Vannier, S.; Carsin-Nicol, B.; Lavoue, S.; Verin, M.; Gauvrit, J.Y.; Le Tulzo, Y.; et al. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Esbroeck, A.C.M.; Janssen, A.P.A.; Cognetta, A.B., 3rd; Ogasawara, D.; Shpak, G.; van der Kroeg, M.; Kantae, V.; Baggelaar, M.P.; de Vrij, F.M.S.; Deng, H.; et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science (NY) 2017, 356, 1084–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.Z.; Persidsky, Y. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J. Leukoc. Biol. 2013, 93, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Small-Howard, A.L.; Shimoda, L.M.; Adra, C.N.; Turner, H. Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells. Biochem. J. 2005, 388, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Lopez, A.J.; Roman-Vega, L.; Ramil Tojeiro, E.; Giuffrida, A.; Garcia-Merino, A. Regulation of cannabinoid receptor gene expression and endocannabinoid levels in lymphocyte subsets by interferon-beta: A longitudinal study in multiple sclerosis patients. Clin. Exp. Immunol. 2015, 179, 119–127. [Google Scholar] [CrossRef] [Green Version]
| Cell Type | Receptor | Species and Used Cells/Tissues in Related Studies |
|---|---|---|
| Alveolar Type-II epithelial cells | CB1 | Human [130] |
| Nerves | CB1 | Rat, Human [89,92] |
| Macrophages | CB1/CB2 | Human CD68(+), CD36(+) macrophages (CD: cluster of differentiation); THP-1 human monocytic cell line-derived macrophages; Murine RAW264.7 macrophage cell line [73] Human lung macrophages; lung cancer-associated macrophages [136] Human monocyte-derived macrophages [137] |
| Mast cells | CB1/CB2 | RBL2H3 mast cell line [58] Human CTS-mast cells [59] Human mucosal type mast cells [60] |
| Eosinophils | CB1/CB2 | EoL-1 cells [69] Human eosinophils from peripheral blood [69,70,71,138] |
| Dendritic cells | CB1/CB2 | Mouse bone marrow-derived dendritic cells [49,80] Human dendritic cells from peripheral blood [79] |
| Neutrophils | CB1/CB2 | Human promyelocytic cell line HL60 [33,81] Human neutrophils from peripheral blood [33,69,70,71,81] |
| B cells | CB1/CB2 | Human B cells from peripheral blood [33,54,74] Human tonsillar B cells [54] Human B lymphoblastoid cell line DAUDI [33] |
| T lymphocytes | CB1/CB2 | Human T cells from peripheral blood [74,139] |
| Basophils | CB1/CB2 | Human basophils from peripheral blood [138] |
| NK cells | CB1/CB2 | Human NK cells from peripheral blood [33] |
| Monocytes | CB1/CB2 | Human monocytes from peripheral blood [33,72,73,74] Human monocytic cell line U937 [33] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozkurt, T.E. Endocannabinoid System in the Airways. Molecules 2019, 24, 4626. https://doi.org/10.3390/molecules24244626
Bozkurt TE. Endocannabinoid System in the Airways. Molecules. 2019; 24(24):4626. https://doi.org/10.3390/molecules24244626
Chicago/Turabian StyleBozkurt, Turgut Emrah. 2019. "Endocannabinoid System in the Airways" Molecules 24, no. 24: 4626. https://doi.org/10.3390/molecules24244626





