Autofluorescence Imaging Reflects the Nuclear Enlargement of Tumor Cells as well as the Cell Proliferation Ability and Aberrant Status of the p53, Ki-67, and p16 Genes in Colon Neoplasms
Abstract
:1. Introduction
2. Results
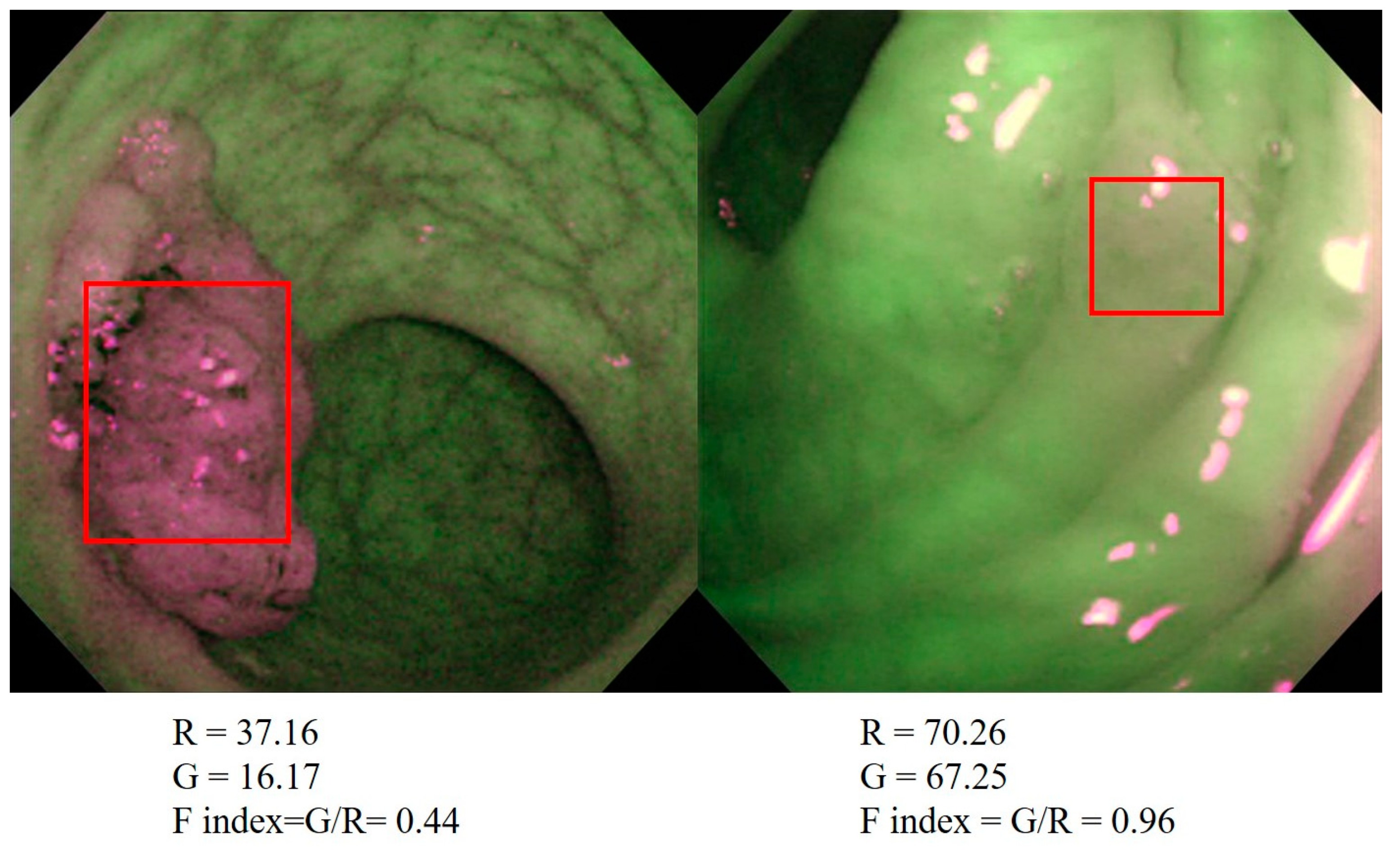
2.1. The Relationship between the F index and the Histological Characteristics of the Neoplasms
2.2. Relationships between the F index, Cell Proliferation Ability, and Aberrant Expression of p53 Proteins
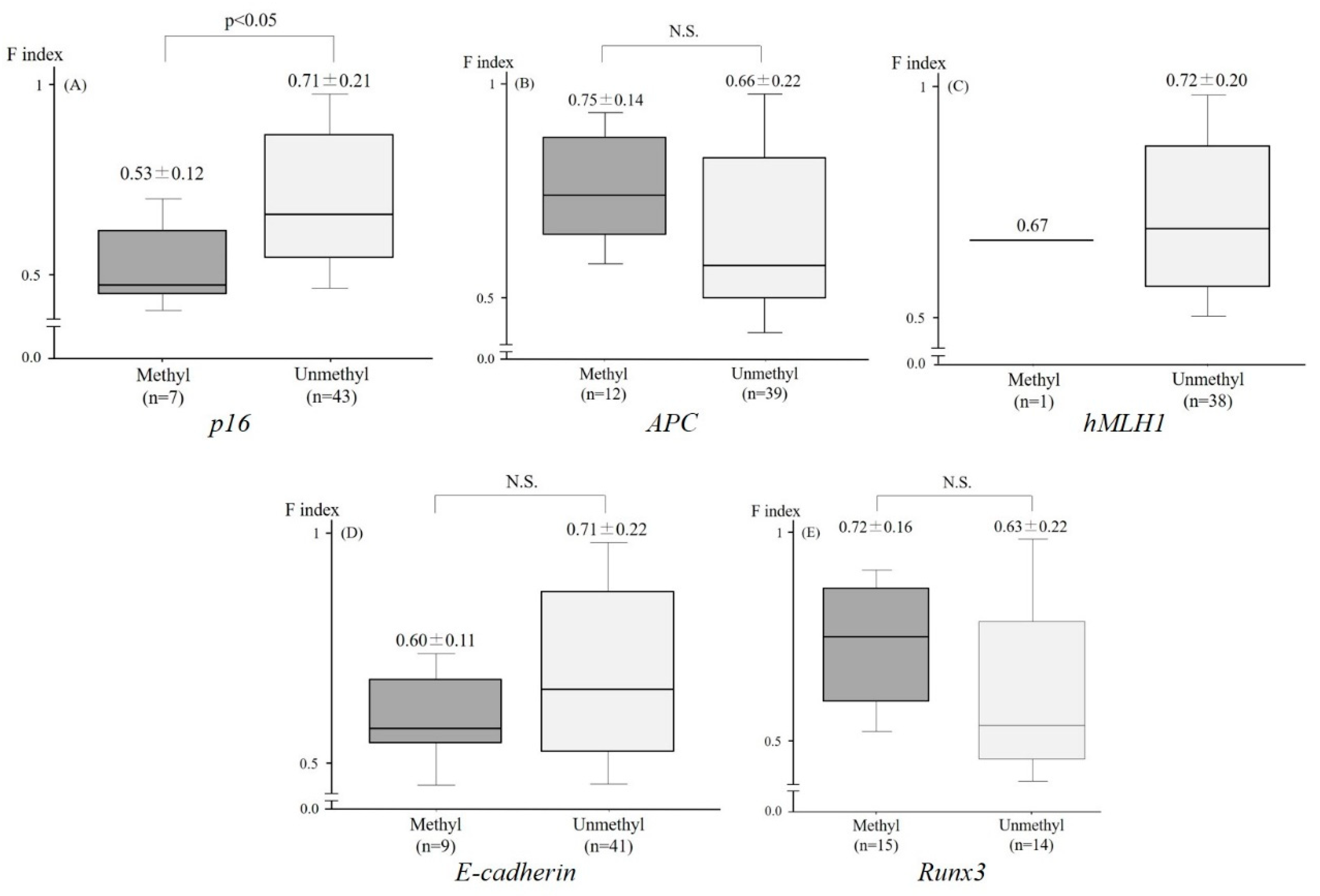
2.3. Relationship between the F index and the Aberrant Methylation of Tumor-Related Genes
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. AFI
4.3. Histological Assessment
4.4. Immunohistochemistry
4.5. DNA Preparation and Methylation-Specific PCR
4.6. Statistical Analyses
Author Contributions
Conflicts of Interest
References
- Fearon, E.R.; Vogelstein, B.A. Genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic instabilities in human cancers. Nature 1998, 396, 643–649. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Eng. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.J.; Fearon, E.R.; Nigro, J.M.; Hamilton, S.R.; Preisinger, A.C.; Jessup, J.M.; van Tuinen, P.; Ledbetter, D.H.; Barker, D.F.; Nakamura, Y.; et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 1989, 244, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kamb, A.; Gruis, N.A.; Weaver-Feldhaus, J.; Liu, Q.; Harshman, K.; Tavtigian, S.V.; Stockert, E.; Day, R.S., 3rd; Johnson, B.E.; Skolnick, M.H. A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994, 264, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltenbach, T.; Sano, Y.; Friedland, S.; Soetikno, R. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology 2008, 134, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Kiesslich, R. Advances in endoscopic imaging of colorectal neoplasia. Gastroenterology 2010, 138, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Fujiya, M.; Kohgo, Y. Image-enhanced endoscopy for the diagnosis of colon neoplasms. Gastrointest. Endosc. 2013, 77, 111–118. [Google Scholar] [CrossRef]
- Moriichi, K.; Fujiya, M.; Sato, R.; Watari, J.; Nomura, Y.; Nata, T.; Ueno, N.; Maeda, S.; Kashima, S.; Itabashi, K.; et al. Back-to-back comparison of auto-fluorescence imaging (AFI) versus high resolution white light colonoscopy for adenoma detection. BMC Gastroenterol. 2012, 12, 75. [Google Scholar] [CrossRef]
- Uedo, N.; Iishi, H.; Tatsuta, M.; Yamada, T.; Ogiyama, H.; Imanaka, K.; Sugimoto, N.; Higashino, K.; Ishihara, R.; Narahara, H.; et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest. Endosc. 2005, 62, 521–528. [Google Scholar] [CrossRef]
- Matsuda, T.; Saito, Y.; Fu, K.I.; Uraoka, T.; Kobayashi, N.; Nakajima, T.; Ikehara, H.; Mashimo, Y.; Shimoda, T.; Murakami, Y.; et al. Does autofluorescence imaging videoendoscopy system improve the colonoscopic polyp detection rate? A pilot study. Am. J. Gastroenterol. 2008, 103, 1926–1932. [Google Scholar] [CrossRef]
- Moriichi, K.; Fujiya, M.; Okumura, T. The efficacy of autofluorescence imaging in the diagnosis of colorectal diseases. Clin. J. Gastroenterol. 2016, 9, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgakoudi, I.; Jacobson, B.C.; Van Dam, J.; Backman, V.; Wallace, M.B.; Müller, M.G.; Zhang, Q.; Badizadegan, K.; Sun, D.; Thomas, G.A.; et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001, 120, 1620–1629. [Google Scholar] [CrossRef]
- Borovicka, J.; Fischer, J.; Neuweiler, J.; Netzer, P.; Gschossmann, J.; Ehmann, T.; Bauerfeind, P.; Dorta, G.; Zürcher, U.; Binek, J.; et al. Autofluorescence endoscopy in surveillance of Barrett’s esophagus: A multicenter randomized trial on diagnostic efficacy. Endoscopy 2006, 38, 867–872. [Google Scholar] [CrossRef]
- Kara, M.A.; Bergman, J.J. Autofluorescence imaging and narrow-band imaging for the detection of early neoplasia in patients with Barrett’s esophagus. Endoscopy 2006, 38, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Messmann, H.; Endlicher, E.; Freunek, G.; Rümmele, P.; Schölmerich, J.; Knüchel, R. Fluorescence endoscopy for the detection of low and high grade dysplasia in ulcerative colitis using systemic or local 5-aminolaevulinic acid sensitisation. Gut 2003, 52, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Moriyama, T.; Yao, T.; Mibu, R.; Iida, M. Autofluorescence imaging colonoscopy for the diagnosis of dysplasia in ulcerative colitis. Inflamm. Bowel Dis. 2007, 13, 640–641. [Google Scholar] [CrossRef]
- Van den Broek, F.J.; Fockens, P.; van Eeden, S.; Reitsma, J.B.; Hardwick, J.C.; Stokkers, P.C.; Dekker, E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: Randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut 2008, 57, 1083–1089. [Google Scholar] [CrossRef]
- Van den Broek, F.J.; van Soest, E.J.; Naber, A.H.; van Oijen, A.H.; Mallant-Hent, R.C.; Böhmer, C.J.; Scholten, P.; Stokkers, P.C.; Marsman, W.A.; Mathus-Vliegen, E.M. Combining autofluorescence imaging and narrow-band imaging for the differentiation of adenomas from non-neoplastic colonic polyps among experienced and non-experienced endoscopists. Am. J. Gastroenterol. 2009, 104, 1498–1507. [Google Scholar] [CrossRef]
- Sato, R.; Fujiya, M.; Watari, J.; Ueno, N.; Moriichi, K.; Kashima, S.; Maeda, S.; Ando, K.; Kawabata, H.; Sugiyama, R.; et al. The diagnostic accuracy of high-resolution endoscopy, autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma. Endoscopy 2011, 43, 862–868. [Google Scholar] [CrossRef]
- Moriichi, K.; Fujiya, M.; Sato, R.; Nata, T.; Nomura, Y.; Ueno, N.; Ishikawa, C.; Inaba, Y.; Ito, T.; Okamoto, K.; et al. Autofluorescence imaging and the quantitative intensity of fluorescence for evaluating the dysplastic grade of colonic neoplasms. Int. J. Colorectal. Dis. 2012, 27, 325–330. [Google Scholar] [CrossRef]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef]
- Bieging, K.T.; Attardi, L.D. Cancer: A piece of the p53 puzzle. Nature 2015, 520, 37–38. [Google Scholar] [CrossRef]
- Merlo, A.; Herman, J.G.; Mao, L.; Lee, D.J.; Gabrielson, E.; Burger, P.C.; Baylin, S.B.; Sidransky, D. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat. Med. 1995, 1, 686–692. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of Apc and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- El Zoghbi, M.; Cummings, L.C. New era of colorectal cancer screening. World J. Gastrointest. Endosc. 2016, 8, 252–258. [Google Scholar] [CrossRef]
- Lamlum, H.; Papadopoulou, A.; Ilyas, M.; Rowan, A.; Gillet, C.; Hanby, A.; Talbot, I.; Bodmer, W.; Tomlinson, I. APC mutations are sufficient for the growth of early colorectal adenomas. Proc. Natl. Acad. Sci. USA 2000, 97, 2225–2228. [Google Scholar] [CrossRef] [Green Version]
- Behrens, J.; Mareel, M.M.; Van Roy, F.M.; Birchmeier, W. Dissecting tumor cell invasion: Epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J. Cell Biol. 1989, 108, 2435–2447. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef]
- Perl, A.K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef]
- Gergen, J.P.; Butler, B.A. Isolation of the Drosophila segmentation gene runt and analysis of its expression during embryogenesis. Genes Dev. 1988, 2, 1179–1193. [Google Scholar] [CrossRef]
- Ku, J.L.; Kang, S.B.; Shin, Y.K.; Kang, H.C.; Hong, S.H.; Kim, I.J.; Shin, J.H.; Han, I.O.; Park, J.G. Promoter hypermethylation downregulates RUNX3 gene expression in colorectal cancer cell lines. Oncogene 2004, 23, 6736–6742. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Arnold, C.N.; Tassone, P.; Chang, D.K.; Niedzwiecki, D.; Dowell, J.M.; Wasserman, L.; Compton, C.; Mayer, R.J.; Bertagnolli, M.M.; et al. Epigenetic inactivation of RUNX3 in microsatellite unstable sporadic colon cancers. Int. J. Cancer 2004, 112, 754–759. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef]
- Lord, M.G.; Valies, O.A. Morphologic study of the submucosa of the large intestine. Surg. Gynecol. Obstet. 1977, 145, 55–60. [Google Scholar]
- Takehana, S.; Kaneko, M.; Mizuno, H. Endoscopic diagnostic system using autofluorescence. Diagn. Ther. Endosc. 1999, 5, 59–63. [Google Scholar] [CrossRef]
- Fujiya, M.; Saitoh, Y.; Watari, J.; Moriichi, K.; Kohgo, Y. Auto-Fluorescence Imaging is useful to assess the activity of ulcerative colitis. Dig. Endosc. 2007, 19, S145–S149. [Google Scholar] [CrossRef]
- Aihara, H.; Sumiyama, K.; Saito, S.; Tajiri, H.; Ikegami, M. Numerical analysis of the autofluorescence intensity of neoplastic and non-neoplastic colorectal lesions by using a novel videoendoscopy system. Gastrointest. Endosc. 2009, 69, 726–733. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Agudo, D.; Gomez-Esquer, F.; Martinez-Arribas, F.; Núñez-Villar, M.J.; Pollán, M.; Schneider, J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. Int. J. Cancer 2004, 109, 717–720. [Google Scholar] [CrossRef]
- Tanaka, A.; Watari, J.; Tanabe, H.; Maemoto, A.; Fujiya, M.; Ashida, T.; Das, K.M.; Kohgo, Y. Effect of eradication of Helicobacter pylori on genetic instabilities in gastric intestinal metaplasia. Aliment. Pharmacol. Ther. 2006, 24, 194–202. [Google Scholar] [CrossRef]
- Shibata, N.; Watari, J.; Fujiya, M.; Tanno, S.; Saitoh, Y.; Kohgo, Y. Cell kinetic and genetic instabilities in differentiated type early gastric cancers with different mucin phenotype. Hum. Pathol. 2003, 34, 32–40. [Google Scholar] [CrossRef]
- Moriichi, K.; Watari, J.; Das, K.M.; Tanabe, H.; Fujiya, M.; Ashida, T.; Kohgo, Y. Effects of Helicobacter pylori infection on genetic instability, the aberrant CpG island methylation status and the cellular phenotype in Barrett’s esophagus in a Japanese population. Int. J. Cancer 2009, 12, 1263–1269. [Google Scholar] [CrossRef]
- Herman, J.G.; Graff, J.R.; Myöhänen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef]
- Sakamoto, J.; Fujiya, M.; Okamoto, K.; Nata, T.; Inaba, Y.; Moriichi, K.; Tanabe, H.; Mizukami, Y.; Watari, J.; Ashida, T.; et al. Immunoprecipitation of nucleosomal DNA is a novel procedure to improve the sensitivity of serum screening for the p16 hypermethylation associated with colon cancer. Cancer Epidemiol. 2010, 34, 194–199. [Google Scholar] [CrossRef]
- Zysman, M.; Saka, A.; Millar, A.; Knight, J.; Chapman, W.; Bapat, B. Methylation of Adenomatous polyposis coli in endometrial cancer occurs more frequently in tumors with microsatellite instability phenotype. Cancer Res. 2002, 62, 3663–3666. [Google Scholar]
- Kang, G.H.; Shim, Y.H.; Ro, J.Y. Correlation of methylation of the hMLH1 promoter with lack of expression of hMLH1 in sporadic gastric carcinomas with replication error. Lab. Investig. 1999, 79, 903–909. [Google Scholar]
- Li, Q.L.; Kim, H.R.; Kim, W.J.; Choi, J.K.; Lee, Y.H.; Kim, H.M.; Li, L.S.; Kim, H.; Chang, J.; Ito, Y.; et al. Transcriptional silencing of RUNX3 gene by CpG hypermethylation is associated with lung cancer. Biochem. Biophys. Res. Commun. 2004, 314, 223–228. [Google Scholar] [CrossRef]
- Szantai, E.; Ronai, Z.; Sasvari-Szekely, M.; Bonn, G.; Guttman, A. Multicapillary electrophoresis analysis of single-nucleotide sequence variations in the deoxycytidine kinase gene. Clin. Chem. 2006, 52, 1756–1762. [Google Scholar] [CrossRef]
Sample Availability: The histological samples of tumors are partially available from the authors. |



| Variables | Regression Coefficient | 95% CI |
|---|---|---|
| Tumor crypt density | −0.072 | −0.346, 0.202 |
| Inflammation cell density | 1.490 | −7.798, 0.778 |
| Tumor cell density | −5.330 | −17.371, 6.710 |
| N/C ratio | −0.522 | −0.960, −0.085 |
| Primer Name | Primer Sequence (5′–3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| hMLH1-U (F) | TTTTGATGTAGATGTTTTATTAGGGTTGT | 124 | 60 |
| hMLH1-U (R) | ACCACCTCATCATAACTACCCACA | ||
| hMLH1-M (F) | TATATCGTTCGTAGTATTCGTGT | 153 | 60 |
| hMLH1-M (R) | TCCGACCCGAATAAACCCAA | ||
| E-cadherin-U (F) | TGGTTGTAGTTATGTATTTATTTTTAGTGGTGTT | 120 | 60 |
| E-cadherin-U (R) | ACACCAAATACAATCAAATCAAACCAAA | ||
| E-cadherin-M (F) | TGTAGTTACGTATTTATTTTTAGTGGCGTC | 112 | 64 |
| E-cadherin-M (R) | CGAATACGATCGAATCGAACCG | ||
| p16-U (F) | TTATTAGAGGGTGGGGTGGATTGT | 151 | 60 |
| p16-U (R) | CAACCCCAAACCACAACCATAA | ||
| p16-M (F) | TTATTAGAGGGTGGGGCGGATCGC | 150 | 65 |
| p16-M (R) | GACCCCGAACCGCGACCGTAA | ||
| APC-U (F) | GTGTTTTATTGTGGAGTGTGGGTT | 108 | 61 |
| APC-U (R) | CCAATCAACAAACTCCCAACAA | ||
| APC-M (F) | TATTGCGGAGTGCGGGTC | 98 | 63 |
| APC-M (R) | TCGACGAACTCCCGACGA | ||
| Runx3-U (F) | TTATGAGGGGTGGTTGTATGTGGG | 221 | 56 |
| Runx3-U (R) | AAAACAACCAACACAAACACCTC | ||
| Runx3-M (F) | TTACGAGGGGCGGTCGTACGCGGG | 221 | 66 |
| Runx3-M (R) | AAAACGACCGACGCGAACGCCTCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriichi, K.; Fujiya, M.; Kobayashi, Y.; Murakami, Y.; Iwama, T.; Kunogi, T.; Sasaki, T.; Ijiri, M.; Takahashi, K.; Tanaka, K.; et al. Autofluorescence Imaging Reflects the Nuclear Enlargement of Tumor Cells as well as the Cell Proliferation Ability and Aberrant Status of the p53, Ki-67, and p16 Genes in Colon Neoplasms. Molecules 2019, 24, 1106. https://doi.org/10.3390/molecules24061106
Moriichi K, Fujiya M, Kobayashi Y, Murakami Y, Iwama T, Kunogi T, Sasaki T, Ijiri M, Takahashi K, Tanaka K, et al. Autofluorescence Imaging Reflects the Nuclear Enlargement of Tumor Cells as well as the Cell Proliferation Ability and Aberrant Status of the p53, Ki-67, and p16 Genes in Colon Neoplasms. Molecules. 2019; 24(6):1106. https://doi.org/10.3390/molecules24061106
Chicago/Turabian StyleMoriichi, Kentaro, Mikihiro Fujiya, Yu Kobayashi, Yuki Murakami, Takuya Iwama, Takehito Kunogi, Takahiro Sasaki, Masami Ijiri, Keitaro Takahashi, Kazuyuki Tanaka, and et al. 2019. "Autofluorescence Imaging Reflects the Nuclear Enlargement of Tumor Cells as well as the Cell Proliferation Ability and Aberrant Status of the p53, Ki-67, and p16 Genes in Colon Neoplasms" Molecules 24, no. 6: 1106. https://doi.org/10.3390/molecules24061106






