Enhanced Supercapacitor Performance Based on CoAl Layered Double Hydroxide-Polyaniline Hybrid Electrodes Manufactured Using Hydrothermal-Electrodeposition Technology
Abstract
:1. Introduction
2. Results and Discussion
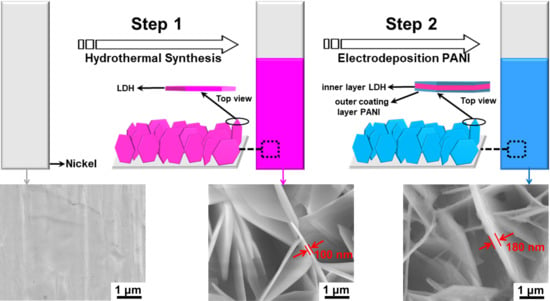
2.1. Structural Study
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Synthesis of the CoAl LDH Nanosheet Structures
3.2. Synthesis of the CoAl LDH-PANI Nanocomposites
3.3. Structural Characterization, Theoretical Calculations and Electrochemical Performance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Stabilized TiN nanowire arrays for high-performance and flexible supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Peng, X.; Liu, B.; Wu, C.; Xie, Y.; Yu, G. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett. 2013, 13, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Song, M.K.; Park, Y.J.; Kim, J.M.; Liu, M.; Wang, Z.L. Fiber supercapacitors made of nanowire-fiber hybrid structures for wearable/flexible energy storage. Angew, Chem. Int. Ed. 2011, 50, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- He, Y.B.; Li, G.R.; Wang, Z.L.; Su, C.Y.; Tong, Y.X. Single-crystal ZnO nanorod/amorphous and nanoporous metal oxide shell composites: Controllable electrochemical synthesis and enhanced supercapacitor performances. Energy Environ. Sci. 2011, 4, 1288–1292. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Wei, J.; Zhou, Z. Preparation and electrochemical performances of doughnut-like Ni(OH)2-Co(OH)2 composites as pseudocapacitor materials. Nanoscale 2012, 4, 4498–4503. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, Z.J.; Li, R.Y.; Ning, Q.; Kong, H.; Niu, Y.L.; Liu, J.K. Nickel-cobalt double hydroxides microspheres with hollow interior and hedgehog-like exterior structures for supercapacitors. J. Mater. Chem. 2012, 22, 23587–23592. [Google Scholar]
- Pan, G.X.; Xia, X.H.; Luo, J.S.; Cao, F.; Yang, Z.H.; Fan, H.J. Preparation of CoAl layered double hydroxide nanoflake arrays and their high supercapacitance performance. Appl. Clay Sci. 2014, 102, 28–32. [Google Scholar]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Huang, J.; Lei, T.; Wei, X.; Liu, X.; Liu, T.; Cao, D.; Yin, J.; Wang, G. Effect of Al-Doped β-Ni (OH)2 nanosheets on electrochemical behaviors for high performance supercapacitor application. J. Power Sources 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Abushrenta, N.; Wu, X.; Wang, J.; Liu, J.; Sun, X. Hierarchical Co-based porous layered double hydroxide arrays derived via alkali etching for high-performance supercapacitors. Sci. Rep. 2015, 5, 13082. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wang, J.; Niu, L.; Sun, J.; Gong, P.; Yang, S. Controllable synthesis of CoAl LDH@Ni(OH)2 nanosheet arrays as binder-free electrode for supercapacitor applications. J. Alloys Compd. 2014, 608, 297–303. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Ling, Z.; Qiu, J.; Gogotsi, Y. Facile fabrication of MWCNT-doped NiCoAl-layered double hydroxide nanosheets with enhanced electrochemical performances. J. Mater. Chem. A 2013, 1, 1963–1968. [Google Scholar] [CrossRef]
- Wang, Y.G.; Cheng, L.; Xia, Y.Y. Electrochemical profile of nano-particle CoAl double hydroxide/active carbon supercapacitor using KOH electrolyte solution. J. Power Sources 2006, 153, 191–196. [Google Scholar] [CrossRef]
- Malak-Polaczyk, A.; Vix-Guterl, C.; Frackowiak, E. Carbon/layered double hydroxide (LDH) composites for supercapacitor application. Energy Fuels 2010, 24, 3346–3351. [Google Scholar] [CrossRef]
- Chen, Z.; Augustyn, V.; Jia, X.; Xiao, Q.; Dunn, B.; Lu, Y. High-performance sodium-ion pseudocapacitors based on hierarchically porous nanowire composites. ACS Nano 2012, 6, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Duay, J.; Lee, S.B. Redox exchange induced MnO2 nanoparticle enrichment in poly (3, 4-ethylenedioxythiophene) nanowires for electrochemical energy storage. ACS Nano 2010, 4, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Z.; Shao, M.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. Flexible hierarchical nanocomposites based on MnO2 nanowires/CoAl hydrotalcite/carbon fibers for high-performance supercapacitors. RSC Adv. 2013, 3, 1045–1049. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Zhao, Y.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Core-shell layered double hydroxide microspheres with tunable interior architecture for supercapacitors. Chem. Mater. 2012, 24, 1192–1197. [Google Scholar] [CrossRef]
- Han, J.; Dou, Y.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Flexible CoAl LDH@PEDOT core/shell nanoplatelet array for high-performance energy storage. Small 2013, 9, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Li, Z.; Zhang, R. Hierarchical Conducting Polymer@Clay Core–Shell Arrays for Flexible All-Solid-State Supercapacitor Devices. Small 2005, 11, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Kutlu, B.; Leuteritz, A.; Boldt, R.; Jehnichen, D.; Wagenknecht, U.; Heinrich, G. PANI-LDH prepared by polymerization-adsorption method and processing to conductive compounds. Appl. Clay Sci. 2013, 72, 91–95. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, T.; Han, B.; Wang, Y.; Du, J.; Liu, Z.; Zhang, J. Aqueous/ionic liquid interfacial polymerization for preparing polyaniline nanoparticles. Polymer 2004, 45, 3017–3019. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef] [Green Version]
- Leng, W.; Zhou, S.; Gu, G.; Wu, L. Wettability switching of SDS-doped polyaniline from hydrophobic to hydrophilic induced by alkaline/reduction reactions. J. Colloid Interface Sci. 2012, 369, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Salaün, F.; Campagne, C. The influence of 1-butanol and trisodium citrate ion on morphology and chemical properties of chitosan-based microcapsules during rigidification by alkali treatment. Mar. Drugs 2014, 12, 5801–5816. [Google Scholar] [CrossRef] [PubMed]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J. Raman Spectrosc. 2004, 35, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Han, J.B.; Lu, J.; Wei, M.; Wang, Z.L.; Duan, X. Heterogeneous ultrathin films fabricated by alternate assembly of exfoliated layered double hydroxides and polyanion. Chem. Commun. 2008, 41, 5188–5190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Angelopoulos, M.; Epstein, A.J. Concentration dependence of aggregation of polyaniline in NMP solution and properties of resulting cast films. Macromolecules 1997, 30, 7634–7637. [Google Scholar] [CrossRef]
- Sinha, S.; Bhadra, S.; Khastgir, D. Effect of dopant type on the properties of polyaniline. J. Appl. Polym. Sci. 2009, 112, 3135–3140. [Google Scholar] [CrossRef]
- Fan, G.; Wang, H.; Xiang, X.; Li, F. Co-Al mixed metal oxides/carbon nanotubes nanocomposite prepared via a precursor route and enhanced catalytic property. J. Solid State Chem. 2013, 197, 14–22. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.J. Green synthesis and characterization of carbon nanotubes/polyaniline nanocomposites. J. Spectrosc. 2015, 2015. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 4, 1597–1614. [Google Scholar] [CrossRef]
- Cooper, S.J.; Bertei, A.; Finegan, D.P. Simulated impedance of diffusion in porous media. Electrochim. Acta 2017, 251, 681–689. [Google Scholar] [CrossRef]
- Chamaani, A.; Safa, M.; Chawla, N. Stabilizing effect of ion complex formation in lithium–oxygen battery electrolytes. J. Electroanal. Chem. 2018, 815, 143–150. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, D.; Zhai, T. Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Yuan, L.; Yao, B.; Hu, B. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Huang, X. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors. Energy Environ. Sci. 2018, 11, 1280–1286. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Bai, H. Degradation-induced capacitance: A new insight into the superior capacitive performance of polyaniline/graphene composites. Energy Environ. Sci. 2017, 10, 2372–2382. [Google Scholar]
- Planes, G.A.; Rodriguez, J.L.; Miras, M.C.; Garcia, G.; Pastor, E.; Barbero, C.A. Spectroscopic evidence for intermediate species formed during aniline polymerization and polyaniline degradation. Phys. Chem. Chem. Phys. 2010, 12, 10584–10593. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, T.; Guo, J. Electrochemical capacity fading of polyaniline electrode in supercapacitor: An XPS analysis. Prog. Nat. Sci. Mater. Int. 2017, 27, 257–260. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, S.; Wu, X. One-Step Electrodeposition of MnO2@NiAl Layered Double Hydroxide Nanostructures on the Nickel Foam for High-Performance Supercapacitors. J. Electrochem. Soc. 2017, 164, 56–62. [Google Scholar] [CrossRef]
- Nakagaki, R.; Frost, D.C.; McDowell, C.A. X-ray photoelectron spectroscopy of nitroanilines and their derivatives. J. Electron Spectrosc. Relat. Phenom. 1981, 22, 289–296. [Google Scholar] [CrossRef]
- Nakagaki, R.; Frost, D.C.; McDowell, C.A. The intramolecular charge-transfer interaction in X-ray photoelectron spectroscopy: The charge-transfer satellites observed in p-nitroaniline and related compounds. J. Electron Spectrosc. Relat. Phenom. 1980, 19, 355–370. [Google Scholar] [CrossRef]
- Nakayama, M.; Saeki, S.; Ogura, K. In situ observation of electrochemical formation and degradation processes of polyaniline by fourier-transform infrared spectroscopy. Anal. Sci. 1999, 15, 259–263. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Q.; Yao, S. A comparative study on polyaniline degradation by an electrochemical quartz crystal impedance system: Electrode and solution effects. Synth. Met. 2004, 143, 119–128. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented- wave method. Phys. Rev. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Adachi, H.; Tsukuda, M.; Satoko, C. Discrete variational Xα cluster calculations. I. Application to metal clusters. J. Phys. Soc. Jpn. 1978, 45, 875–883. [Google Scholar] [CrossRef]
- Muralidharan, N.; Westover, A.S.; Sun, H. From the Junkyard to the Power Grid: Ambient Processing of Scrap Metals into Nanostructured Electrodes for Ultrafast Rechargeable Batteries. ACS Energy Lett. 2016, 1, 1034–1041. [Google Scholar] [CrossRef]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |













© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Takei, T.; Yanagida, S.; Kumada, N. Enhanced Supercapacitor Performance Based on CoAl Layered Double Hydroxide-Polyaniline Hybrid Electrodes Manufactured Using Hydrothermal-Electrodeposition Technology. Molecules 2019, 24, 976. https://doi.org/10.3390/molecules24050976
Yang G, Takei T, Yanagida S, Kumada N. Enhanced Supercapacitor Performance Based on CoAl Layered Double Hydroxide-Polyaniline Hybrid Electrodes Manufactured Using Hydrothermal-Electrodeposition Technology. Molecules. 2019; 24(5):976. https://doi.org/10.3390/molecules24050976
Chicago/Turabian StyleYang, Guoshen, Takahiro Takei, Sayaka Yanagida, and Nobuhiro Kumada. 2019. "Enhanced Supercapacitor Performance Based on CoAl Layered Double Hydroxide-Polyaniline Hybrid Electrodes Manufactured Using Hydrothermal-Electrodeposition Technology" Molecules 24, no. 5: 976. https://doi.org/10.3390/molecules24050976