Use of Nutraceuticals in Angiogenesis-Dependent Disorders
Abstract
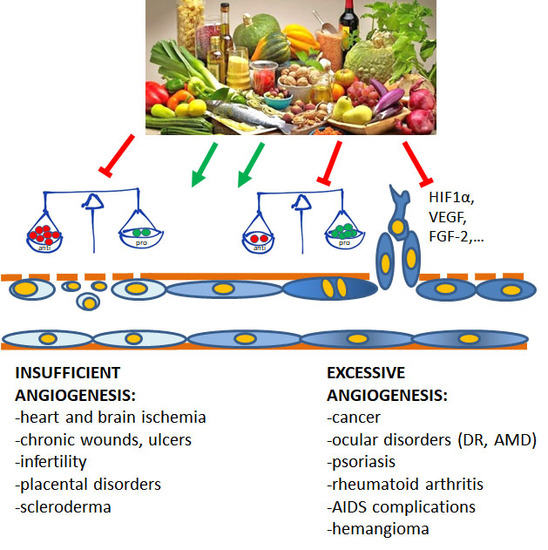
:1. Angiogenesis Definition and Mechanisms
2. Angiogenesis Related Diseases
3. Nutraceuticals
3.1. Nutraceutical Antiangiogenic Strategies
3.1.1. Chemopreventive and Antitumor Approaches
3.1.2. Nutraceuticals and Ocular Disorders
3.2. Nutraceuticals and Pro-Endothelium Applications
3.2.1. Interventions for Endothelial Dysfunction
3.2.2. Nutraceutical Approaches for Diabetes Mellitus
3.2.3. Nutraceutical Control of Placenta Development and Preeclampsia
3.2.4. Vascular Ageing and Cerebrovascular Interventions
3.2.5. Biomaterials Biocompatibility/Integration and Wound Healing
4. Pharmacological Issues to Be Solved
5. Conclusions
Funding
Conflicts of Interest
References
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases: From genes to function to therapy. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Schneider, M.; Carmeliet, P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb. Exp. Pharmacol. 2006, 176, 157–212. [Google Scholar]
- Ziche, M.; Jones, J.; Gullino, P.M. Role of prostaglandin E1 and copper in angiogenesis. J. Natl. Cancer Inst. 1982, 69, 475–482. [Google Scholar] [PubMed]
- Finetti, F.; Solito, R.; Morbidelli, L.; Giachetti, A.; Ziche, M.; Donnini, S. Prostaglandin E2 regulates angiogenesis via activation of fibroblast growth factor receptor-1. J. Biol. Chem. 2008, 283, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Masini, E.; Amerini, S.; Granger, H.J.; Maggi, C.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994, 94, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L. Molecular regulation of tumor angiogenesis by nitric oxide. Eur. Cytokine Netw. 2009, 20, 164–170. [Google Scholar] [PubMed]
- Parenti, A.; Morbidelli, L.; Ledda, F.; Granger, H.J.; Ziche, M. The bradykinin/B1 receptor promotes angiogenesis by upregulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. FASEB J. 2001, 15, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Parenti, A.; Ledda, F. Nitric oxide modulates angiogenesis elicited by prostaglandin E1 in rabbit cornea. In Advances in Prostaglandins, Thromboxane, and Leukotriene Research; Samuelsson, B., Paoletti, R., Ramwell, P.W., Eds.; Raven Press: New York, NY, USA, 1995; Volume 23, pp. 495–497. [Google Scholar]
- Morbidelli, L.; Chang, C.-H.; Douglas, J.G.; Granger, H.J.; Ledda, F.; Ziche, M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am. J. Physiol. 1996, 270, H411–H415. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Keaney, J.F., Jr.; Hunter, L.M.; Watkins, M.T.; Menzoian, J.O.; Vita, J.A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002, 105, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Muñoz-Chápuli, R.; Quesada, A.R. Challenges of antiangiogenic cancer therapy: Trials and errors, and renewed hope. J. Cell. Mol. Med. 2007, 11, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.R.; Muñoz-Chápuli, R.; Medina, M.A. Anti-angiogenic drugs: From bench to clinical trials. Med. Res. Rev. 2006, 26, 483–530. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.R.; Medina, M.A.; Muñoz-Chápuli, R.; Ponce, A.L.G. Do not say ever never more: The ins and outs of antiangiogenic therapies. Curr. Pharm. Des. 2010, 16, 3932–3957. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Nakagami, H.; Koriyama, H.; Morishita, R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. Biomed. Res. Int. 2013, 2013, 186215. [Google Scholar] [CrossRef] [PubMed]
- Besnier, M.; Gasparino, S.; Vono, R.; Sangalli, E.; Facoetti, A.; Bollati, V.; Cantone, L.; Zaccagnini, G.; Maimone, B.; Fuschi, P.; et al. MiR-210 enhances the therapeutic potential of bone-marrow-derived circulating proangiogenic cells in the setting of limb ischemia. Mol. Ther. 2018, 26, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Williams, J.O.; Ramji, D.P. Nutraceuticals as therapeutic agents for atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Souyoul, S.A.; Saussy, K.P.; Lupo, M.P. Nutraceuticals: A Review. Dermatol. Ther. 2018, 8, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann. Ist. Super Sanità 2007, 43, 348–361. [Google Scholar] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies 1–4. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.P.; Wang, A.; Ye, J.H.; Zheng, X.Q.; Polito, C.A.; Lu, J.L.; Li, Q.S.; Liang, Y.R. Suppressive effects of tea catechins on breast cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Sergiu, C.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary intervention by phytochemicals and their role in modulating coding and non-coding genes in cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cheng, J.; Wang, X. Dietary antioxidants: Potential anticancer agents. Nutr. Cancer 2017, 69, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Tsai, C.M.; Lu, C.C.; Weng, C.J. Recent progress in natural dietary non-phenolic bioactives on cancers metastasis. J. Food Drug Anal. 2018, 26, 940–964. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L. Polyphenol-based nutraceuticals for the control of angiogenesis: Analysis of the critical issues for human use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.; Suliburska, J.; Ferreira, I.M.P.L.V.O. New insights into the antiangiogenic and proangiogenic properties of dietary polyphenols. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.L.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Li, V.W.; Hutnik, M.; Chiou, A.S. Tumor angiogenesis as a target for dietary cancer prevention. J. Oncol. 2012, 2012, 879623. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Gupta, P.; Singh, B.; Koul, A. Lycopene enriched tomato extract inhibits hypoxia, angiogenesis, and metastatic markers in early stage n-nitrosodiethylamine induced hepatocellular carcinoma. Nutr. Cancer 2015, 67, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Kowshik, J.; Krishnaraj, J.; Sophia, J.; Dixit, M.; Nagini, S. Blueberry inhibits invasion and angiogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters via suppression of TGF-β and NF-κB signaling pathways. J. Nutr. Biochem. 2016, 35, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.C.; Tomé-Carneiro, J.; Alberto Dávalos, A.; Visioli, F. Pharma-nutritional properties of olive oil phenols. transfer of new findings to human nutrition. Foods 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Donnini, S.; Giachetti, A.; Iniguez, M.A.; Fresno, M.; Melillo, G.; Ziche, M. Inhibition of hypoxia inducible factor-1alpha by dihydroxyphenylethanol, a product from olive oil, blocks microsomal prostaglandin-E synthase-1/vascular endothelial growth factor expression and reduces tumor angiogenesis. Clin. Cancer Res. 2010, 16, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Nannelli, G.; Frosini, M.; Giachetti, A.; Ziche, M.; Donnini, S. Inhibition of cell cycle progression by the hydroxytyrosol-cetuximab combination yields enhanced chemotherapeutic efficacy in colon cancer cells. Oncotarget 2017, 8, 83207–83224. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lim, D.Y.; Jung, J.I.; Cho, H.J.; Park, S.Y.; Kwon, G.T.; Kang, Y.H.; Lee, K.W.; Choi, M.S.; Park, J.H.Y. Dietary oleuropein inhibits tumor angiogenesis and lymphangiogenesis in the B16F10 melanoma allograft model: A mechanism for the suppression of high-fat diet-induced solid tumor growth and lymph node metastasis. Oncotarget 2017, 8, 32027–32042. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R.; Anupam, A.; Manchikanti, P.; Rameshbabu, A.P.; Dasgupta, S.; Dhara, S. Identification and characterization of bioactive phenolic constituents, anti-proliferative, and anti-angiogenic activity of stem extracts of Basella alba and rubra. J. Food Sci. Technol. 2018, 55, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Shakya, G.; Balasubramanian, S.; Hoda, M.; Rajagopalan, R. Inhibition of metastasis and angiogenesis in Hep-2 cells by wheatgrass extract—An in vitro and in silico approach. Toxicol. Mech. Methods 2018, 28, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.T.; Stan, S.D. Dietary bioactive diallyl trisulfide in cancer prevention and treatment. Int. J. Mol. Sci. 2017, 18, 1645. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.H. Anticancer Properties of Capsaicin against Human Cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Medina, M.A.; Quesada, A.R. Dietary proteins and angiogenesis. Nutrients 2014, 6, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, M.; Luo, C.C.; Wang, J.Q.; Zheng, N. lactoferrin exerts antitumor effects by inhibiting angiogenesis in a HT29 human colon tumor model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and ophthalmic diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.; Chen, S.; Uchi, S.H.; Nanri, T.; Connor, K.M.; Kimura, K. Attenuation of choroidal neovascularization by dietary intake of ω-3 long-chain polyunsaturated fatty acids and lutein in mice. PLoS ONE 2018, 13, e0196037. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal protection and distribution of curcumin in vitro and in vivo. Front. Pharmacol. 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Naidoo, N.; van Dam, R.M. Diet and endothelial function: From individual components to dietary patterns. Curr. Opin. Lipidol. 2012, 23, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Predimed study investigators. primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra virgin olive oil and cardiovascular diseases: Benefits for human health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Caliceti, C.; Fogacci, F.; Giovannini, M.; Calabria, D.; Colletti, A.; Veronesi, M.; Roda, A.; Borghi, C. Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Stanhewicz, A.E.; Kenney, W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Yang, K.Q.; Cui, J.G.; Zhou, L.L.; Zhou, X.L. Folic Acid Supplementation for Stroke Prevention in Patients with Cardiovascular Disease. Am. J. Med. Sci. 2017, 354, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Borgi, L.; McMullan, C.; Wohlhueter, A.; Curhan, G.C.; Fisher, N.D.; Forman, J.P. Effect of Vitamin D on endothelial function: A randomized, double-blind, placebo-controlled trial. Am. J. Hypertens 2017, 30, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Cazeau, R.M.; Huang, H.; Bauer, J.A.; Hoffman, R.P. Effect of Vitamins C and E on Endothelial Function in Type 1 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 3271293. [Google Scholar] [CrossRef] [PubMed]
- Zarei, F.; Negahdari, B.; Eatemadi, A. Diabetic ulcer regeneration: Stem cells, biomaterials, growth factors. Artif. Cells Nanomed. Biotechnol. 2018, 46, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.C.; Peng, L.S.; Zou, L.; Huang, S.F.; Xie, Y.; Mu, G.P.; Zeng, X.H.; Zhou, X.L.; Zeng, Y.C. Protective effect and mechanism of lycopene on endothelial progenitor cells (EPCs) from type 2 diabetes mellitus rats. Biomed. Pharmacother. 2017, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.A.; Bhonde, R.R. Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol. Res. 2018, 132, 90–98. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.; Audette, M.C.; Parker, J.D.; Kingdom, J.C. Mechanisms and clinical significance of endothelial dysfunction in high-risk pregnancies. Can. J. Cardiol. 2018, 34, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Németh, B.; Murányi, E.; Hegyi, P.; Mátrai, P.; Szakács, Z.; Varjú, P.; Hamvas, S.; Tinusz, B.; Budán, F.; Czimmer, J.; et al. Asymmetric dimethylarginine levels in preeclampsia—Systematic review and meta-analysis. Placenta 2018, 69, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kemse, N.; Sundrani, D.; Kale, A.; Joshi, S. Maternal Micronutrients, Omega-3 fatty acids and gene expression of angiogenic and inflammatory markers in pregnancy induced hypertension rats. Arch. Med. Res. 2017, 48, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Kemse, N.G.; Kale, A.A.; Joshi, S.R. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension in Wistar rats improves IL10 and VEGF levels. PLEFA 2016, 104, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Accardi, G.; Aiello, A.; Gambino, C.M.; Virruso, C.; Caruso, C.; Candore, G. Mediterranean nutraceutical foods: Strategy to improve vascular ageing. Mech. Ageing Dev. 2016, 159, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Degens, H.; Vanhees, L.; Austin, C.; Azzawi, M. The effects of resveratrol on aging vessels. Exp. Gerontol. 2016, 85, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Wong, R.H.X.; Howe, P.R.C. Effects of long-chain omega-3 polyunsaturated fatty acids on endothelial vasodilator function and cognition-are they interrelated? Nutrients 2017, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Forbe, S.C.; Holroyd-Leduc, J.M.; Poulin, M.J.; Hogan, D.B. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. Geriatr. J. 2015, 18, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Jiang, X.; Hu, X.; Xia, J.; Hong, D.; Zhang, W.; Gao, Y.; Chen, J.; Shi, Y. Delayed docosahexaenoic acid treatment combined with dietary supplementation of omega-3 fatty acids promotes long-term neurovascular restoration after ischemic stroke. Transl. Stroke Res. 2016, 7, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Blatchley, M.R.; Duh, E.J.; Gerecht, S. Acellular and cellular approaches to improve diabetic wound healing. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin tissue substitutes and biomaterial risk assessment and testing. Front. Bioeng. Biotechnol. 2018, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.; Duraipandy, N.; Srivatsan, K.V.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Fabrication of hybrid collagen aerogels reinforced with wheat grass bioactives as instructive scaffolds for collagen turnover and angiogenesis for wound healing applications. ACS Appl. Mater. Interfaces 2017, 9, 16939–16950. [Google Scholar] [CrossRef] [PubMed]
- Dharunya, G.; Duraipandy, N.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Curcumin cross-linked collagen aerogels with controlled anti-proteolytic and pro-angiogenic efficacy. Biomed. Mater. 2016, 11, 045011. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.R.; Shao, Z.M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.; Mathur, P.S.; Greene, G.L. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res. Treat. 2009, 117, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin. Hemorheol. Microcirc. 2006, 34, 109–115. [Google Scholar] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed. Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Fang, J.; Xia, C.; Shi, X.; Jiang, B.H. trans-3,4,5′-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin. Cancer Res. 2004, 10, 5253–5263. [Google Scholar] [CrossRef] [PubMed]
- Brakenhielm, E.; Cao, R.; Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001, 15, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Durrani, A.I.; Schwartz, H.; Nagl, M.; Sontag, G. Determination of free [alpha]-lipoic acid in foodstuffs by HPLC coupled with CEAD and ESI-MS. Food Chem. 2010, 120, 38329–38336. [Google Scholar] [CrossRef]
- Dworacka, M.; Chukanova, G.; Iskakova, S.; Kurmambayev, Y.; Wesołowska, A.; Frycz, B.A.; Jagodziński, P.P.; Dworacki, G. New arguments for beneficial effects of alpha-lipoic acid on the cardiovascular system in the course of type 2 diabetes. Eur. J. Pharm. Sci. 2018, 117, 41–47. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture, Agricultural Research Service, USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 25 August 2018).
- Reule, C.A.; Goyvaerts, B.; Schoen, C. Effects of an l-arginine-based multi ingredient product on endothelial function in subjects with mild to moderate hypertension and hyperhomocysteinemia—A randomized, double-blind, placebo-controlled, cross-over trial. BMC Complement. Altern. Med. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Nassar, F.; Masser, G.; Grosovski, M. Olive oil consumption and non-alcoholic fatty liver disease. World J. Gastroenterol. 2009, 15, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [Green Version]
- Gou, M.; Men, K.; Shi, H.; Xiang, M.; Zhang, J.; Song, J.; Long, J.; Wan, Y.; Luo, F.; Zhao, X.; et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 2011, 3, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Antal, D.S.; Ardelean, F.; Chis, A.R.; Ollivier, E.; Serban, M.-C. Nanoscale delivery systems: Actual and potential applications in the natural products industry. Curr. Pharm. Des. 2017, 23, 2414–2421. [Google Scholar] [CrossRef]
- Donnini, S.; Finetti, F.; Lusini, L.; Morbidelli, L.; Cheynier, V.; Barron, D.; Williamson, G.; Waltenberger, J.; Ziche, M. Divergent effects of quercetin conjugates on angiogenesis. Br. J. Nutr. 2006, 95, 1016–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, P.C.; Pathak, S.; Kumar, V.; Panda, B.P. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology 2018, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Baron-Menguy, C.; Bocquet, A.; Guihot, A.L.; Chappard, D.; Amiot, M.J.; Andriantsitohaina, R.; Loufrani, L.; Henrion, D. Effects of red wine polyphenols on postischemic neovascularization model in rats: Low doses are proangiogenic, high doses anti-angiogenic. FASEB J. 2007, 21, 3511–3521. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, T.; Barik, S.; Das, A.; Ghosh, S.; Bhuniya, A.; Bose, A.; Baral, R. Neem leaf glycoprotein prophylaxis transduces immune dependent stop signal for tumor angiogenic switch within tumor microenvironment. PLoS ONE 2014, 9, e110040. [Google Scholar] [CrossRef] [PubMed]

| Identifier | Active Principle (Dose, Route) | Title of the Study (Status) |
|---|---|---|
| NCT01521949 | Acai Juice 2 ounces by mouth twice daily on a continuous basis | A Phase 2 Study Acai Juice Product in Asymptomatic or Minimally Symptomatic Prostate Cancer Patients With Rising Prostate Specific Antigen (PSA) (Completed, Has results) |
| NCT03665922 | Four tablets (BroccoMax® or placebo) in the morning with breakfast and four tablets in the evening with dinner for daily internal dose of 64 mg of SFN. Four weeks treatment. | Biomarkers of Sulforaphane/Broccoli Sprout Extract in Prostate Cancer (New, Not yet recruiting) |
| NCT01916239 | Two new pomegranate formulations vs. standard pomegranate extract formulation containing 20% punicalagin; 15 days treatment. | Phase I-II Study of Pomegranate Extract Formulations in Colorectal Cancer Patients: Metabolic and Gene Expression Profiling in Tumoral and Normal Colon Tissues (Completed) |
| NCT02984813 | Two pills once daily in the morning for 3 months containing alpha lipoic acid, citicoline, co-enzyme Q10, Ginkgo biloba extract, grape seed extract, N-acetyl-cysteine, curcumin, and green tea extract or curcumin, bilberry extract, and grape seed extract | Safety and Efficacy of Anti-Oxidants and Anti-inflammatory Agents in Glaucoma and Diabetic Retinopathy (Active, not recruiting) |
| NCT01646047 | Two capsules containing nutritional supplements per day for 6 months (vitamin C, mixed tocopherols/tocotrienols, vitamin D, fish oil, lutein, zeaxanthin, pine bark extract, benfotiamine, green tea extract, curcumin) | Diabetes Visual Function Supplement Study (Completed) |
| NCT03676309 | Nutriceutical Oral Capsule, nutraceutical 920 mg (Bergamot 450 mg, Gymnema 400 mg, Phaseolamine 30 mg, Olea Europaea 10 mg) twice day, 12 weeks | Efficacy and Safety of Nutraceuticals in Patients With Diabetes Mellitus Type II and Dyslipidemia. (Completed) |
| NCT03593135 | 15 mL apple cider vinegar (American garden organic vinegar) (containing 5% acetic acid) mixed in 200 mL water during meal at night time (daily, for 3 months) | Effect of Apple Cider Vinegar in Type 2 Diabetics. (Completed) |
| NCT02969070 | LopiGLIK™, Akademy Pharma, 1 capsule/day containing red yeast rice 220 mg (at least 3.3 mg of Monacolin K) + Berberine 531.25 mg + Morus Alba 200 mg (at least 4 mg of Deoxynojirimycin) vs. Armolipid Plus®, Meda Pharma, 1 capsule/day containing Berberis aristata d.e. 588 mg (equivalent to Berberine chloride 500 mg) + Red yeast rice 200 mg (equivalent to Monacolin K 3 mg) + Policosanol 10 mg + Folic acid 0.2 mg + Coenzyme Q10 2.0 mg + Astaxanthin 0.5 mg (Daily for 4 weeks) | Effects of Nutraceutical Therapies on Endothelial Function, Platelet Aggregation, and Coronary Flow Reserve (Recruiting) |
| NCT02772887 | Oral l-citrulline, 3 g once per day for 3 weeks | Nutraceutical Citrulline in Pregnancy (Recruiting) |
| NCT02629952 | Three cups of blueberry tea per day for 4 weeks | Metabolic Benefits of Drinking Blueberry Tea in Type 2 Diabetes (Recruiting) |
| NCT02029833 | Regular Canola Oil 60% or 70% oleic acid (daily for 6 weeks) | Canola Oil Multi-Centre Intervention Trial II (COMIT2) (Completed) |
| NCT01982734 | 80 mg curcumin were given orally either as native powder, native powder plus phytochemicals, micelles or micelles plus phytochemicals (Pharmacokinetics studies) | Improved Oral Bioavailability of Curcumin Incorporated Into Micelles (completed) |
| NCT01925287 | 500 mg curcumin were given orally either as native powder, micronized powder, or liquid micelles (early phase I) | Oral Bioavailability of Curcumin From Micronized Powder and Liquid Micelles in Healthy Young Women and Men (Completed) |
| NCT01449110 | Resveratrol-enriched grape extract (8 mg) (orally, daily for 6 months) | Resveratrol-enriched Grape Extract (Stilvid) in Primary and Secondary Prevention of Cardiovascular Disease (Completed) |
| NCT01085019 | 2.8 g/day of cinnamon/oregano/ginger/rosemary/black pepper in capsules during 4 weeks. | Impact of Spices and Herbs on Endothelial Function (Completed) |
| NCT00296595 | 2 g/day of fish oil + 500 mL/day of cranberry juice (daily for 12 weeks) | Effects of n-3 Polyunsaturated Fatty Acids and Antioxidants on Postprandial Hyperlipidemia and Vascular Function in Men (Completed) |
| NCT00654459 | Mixture of berberine, policosanol, red yeast, placebo. A tablet one a day for 6 weeks | Effects of Armolipid Plus on Cholesterol Levels and Endothelial Function (Completed) |
| Category | Food | Active Principles |
|---|---|---|
| Beverages and drinks | Green tea, red wine | Stilbenoids (resveratrol), flavanols (catechins) |
| Fruits | Strawberries, blackberries, raspberries, blueberries, cranberries, apple, pineapple, cherries, oranges, grapefruit, lemons, red grapes, pomegranate | Carotenoids (lycopene), the most part of flavonoids and in particular glycosides of anthocynidins (anthocyanins), stilbenoids (resveratrol), flavanones (hesperetin), |
| Vegetables and mushrooms | Soy beans, artichokes, tomatoes, garlic, kale, broccoli, cauliflower, Brussels sprouts, bok choy, lavender, maitake mushrooms, parsley, pumpkin | Flavones (apigenin), isoflavones (genistein), flavonols (quercetin), isothiocyanate (sulfurafane), glycosides of anthocyanidins (anthocyanins) |
| Oils | Extra-virgin olive oil, grapeseed oil | Oleic acid, phenylethanoids (hydroxytyrosol) |
| Other | Dark chocolate, ginseng, licorice, turmeric, ginger, nutmeg, cinnamon, red propolis | Glycosides of anthocyanidins (anthocyanins), ginsenoides, phenolic acids (curcumin) |
| Fish and meat | Tuna, sea cucumber | Omega-3 fatty acids, mucopolysaccharides, saponins |
| Categories | Food | Active Principles |
|---|---|---|
| Beverages and drinks | Red wine, grape juice, chocolate, green tea, orange juice | Stilbenoids (resveratrol), flavanols (catechins), vitamins |
| Fruits | Avocados, tomatoes, watermelon, grapefruit, Citrus plants and in general all fruits | Carotenoids (lycopene), glycosides of anthocynidins (anthocyanins), flavanones (hesperetin), vitamins C and E |
| Vegetables | Leafy greens, soybeans, legumes, red clover, flax, alfalfa, Cruciferae family, onions, shallots, garlic | Flavones (luteolin, apigenin), isoflavones (genistein), flavanols (quercetin), isothiocyanate (sulfurafane), glycosides of anthocyanidins (anthocyanins), vitamins C and E, folate, l-arginine (from plant proteins) |
| Oils | Olive oil, flaxseed oil, canola oil, soybean oil, cod liver oil, herring oil, salmon oil | Oleic acid, phenylethanoids (oleuropein, hydroxytyrosol, tyrosol), omega-3 fatty acids |
| Other | Nuts, cereals, grains, | Vitamin E |
| Fish | Anchovy, bass, bluefish, capelin, dogfish, eel, herring, mackerel, mullet, rockfish, sablefish, salmon, saury, scad, smelt, sturgeon, trout, tuna, whitefish | Omega-3 fatty acids |
| Chemical Formula | Nutraceutical [Food Source] | Average Concentration | Reference for Biological Activity |
|---|---|---|---|
| Antiangiogenic nutraceuticals | |||
 | Catechins (flavanol) [green tea, chocolate] | 30–250 mg/kg fresh weight 60–800 mg/L infusion [83,84] | [85,86,87] |
 | Curcumin [Curcuma longa] | 3.14 g/100 g of turmeric powder [83,88] | [89,90] |
 | Resveratrol [grapes, berries, peanuts, etc.] | 0.3–7 mg aglycones/L and 15 mg glycosides/L in red wine [83,84] | [91,92] |
| Proangiogenic/endothelial protective nutraceuticals | |||
 | Alpha lipoic acid [kidney, heart, liver, spinach, broccoli, and yeast extract] [93] | 0.1–2.6 mg/kg dry weight | [94] |
 | l-arginine [turkey breast, pork loin, chicken, pumpkin seed, soybean, peanuts, spirulina, dairy, chickpeas, lentils] [95] | 0.1–3.13 g/100 g of plant food 0.08–1.74 g/100 g of animal food | [96] |
 Alpha-linolenic acid | ω3-PUFA [fish oil, flaxseed oil, canola oil, soybean oil, olive oil] [97,98] | 1–5 mg/100 g of fish 7 mg/tablespoon of flaxseed oil 7.9 g/100 g olive oil | [75,76,77] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morbidelli, L.; Terzuoli, E.; Donnini, S. Use of Nutraceuticals in Angiogenesis-Dependent Disorders. Molecules 2018, 23, 2676. https://doi.org/10.3390/molecules23102676
Morbidelli L, Terzuoli E, Donnini S. Use of Nutraceuticals in Angiogenesis-Dependent Disorders. Molecules. 2018; 23(10):2676. https://doi.org/10.3390/molecules23102676
Chicago/Turabian StyleMorbidelli, Lucia, Erika Terzuoli, and Sandra Donnini. 2018. "Use of Nutraceuticals in Angiogenesis-Dependent Disorders" Molecules 23, no. 10: 2676. https://doi.org/10.3390/molecules23102676