Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer
Abstract
:1. Introduction
2. Results
2.1. Kaempferol Suppresses 2,20-azobis (2-amidinopropane)(AAPH)-Induced Oxidative Damage in Human Erythrocytes
2.2. Antiproliferative Effect of Kaempferol on Bladder Cancer Cells
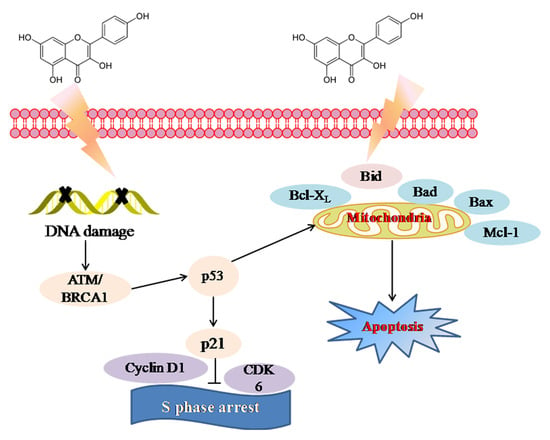
2.3. Molecular Mechanism of Kaempferol-Induced Apoptosis and S Phase Arrest in EJ Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Erythrocyte Hemolytic Assay Mediated by AAPH
4.3. Scanning Electron Microscope (SEM) Observation
4.4. Determination of ROS Generation, MDA Content, and Enzyme Activities of SOD and Gpx
4.5. Culture and Cell Viability Assay
4.6. Flow Cytometric Analysis
4.7. Western Blotting Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Garg, M. Urothelial cancer stem cells and epithelial plasticity: Current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015, 34, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; Athira, K.V.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, G.; Pan, J.; Gong, D. Novel insights into the inhibitory mechanism of kaempferol on xanthine oxidase. J. Agric. Food Chem. 2015, 63, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, X.; Li, C.; Zhao, T.; Hai, J.; Fang, W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumour Biol. 2016, 37, 10247–10256. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nothlings, U.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Flavonols and pancreatic cancer risk: The multiethnic cohort study. Am. J. Epidemiol. 2007, 166, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Morgenstern, H.; Greenland, S.; Tashkin, D.P.; Mao, J.T.; Cai, L.; Cozen, W.; Mack, T.M.; Lu, Q.Y.; Zhang, Z.F. Dietary flavonoid intake and lung cancer—A population-based case-control study. Cancer 2008, 112, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Inserra, F.; Martínezmaldonado, M. Inflammation and the metabolic syndrome: Role of angiotensin II and oxidative stress. Curr. Hypertens. Rep. 2006, 8, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cimen, M.Y. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Dumaswala, U.J.; Zhuo, L.; Jacobsen, D.W.; Jain, S.K.; Sukalski, K.A. Protein and lipid oxidation of banked human erythrocytes: Role of glutathione. Free Radic. Biol. Med. 1999, 27, 1041. [Google Scholar] [CrossRef]
- Miele, L.; Gabrieli, M.L.; Forgione, A.; Vero, V.; Gallo, A.; Capristo, E.; Gasbarrini, G.; Grieco, A. Oxidative stress in metabolic syndrome and nonalcoholic steatohepatitis. Is it possible a role for vitamins in clinical practice? Recenti Prog. Med. 2006, 97, 1–5. [Google Scholar] [PubMed]
- Abraham, A.G.; O’Neill, E. PI3K/Akt-mediated regulation of p53 in cancer. Biochem. Soc. Trans. 2014, 42, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, S.; Tao, R.; Huang, J.; He, X.; Qu, L.; Fu, Z. Oral exposure of mice to cadmium (II), chromium (VI) and their mixture induce oxidative- and endoplasmic reticulum-stress mediated apoptosis in the livers. Environ. Toxicol. 2014, 31, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Jiang, J.; Yin, X.; Wong, K.H.; Zheng, W.; Chen, T. Purification of selenium-containing allophycocyanin from selenium-enriched Spirulina platensis and its hepatoprotective effect against t-BOOH-induced apoptosis. Food Chem. 2012, 134, 253–261. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Desideri, E.; Cardaci, S.; Graziani, I.; Piccirillo, S.; Rotilio, G.; Ciriolo, M.R. Carcinoma cells activate AMP-activated protein kinase-dependent autophagy as survival response to kaempferol-mediated energetic impairment. Autophagy 2010, 6, 202–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.M. Kaempferol protects MC3T3-E1 cells through antioxidant effect and regulation of mitochondrial function. Food Chem. Toxicol. 2011, 49, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Vellosa, J.C.R.; Regasini, L.O.; Khalil, N.M.; da Silva Bolzani, V.; Khalil, O.A.K.; Manente, F.A.; Netto, H.P.; de Faria Oliveira, O.M.M. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Química 2011, 36, 7–20. [Google Scholar] [CrossRef]
- Zhang, T.T.; Lu, C.L.; Jiang, J.G. Antioxidant and anti-tumour evaluation of compounds identified from fruit of Amomum tsaoko Crevost et Lemaire. J. Funct. Foods. 2015, 18, 423–431. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol modulates DNA methylation and downregulates DNMT3B in bladder cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mehta, S.L.; Li, P.A. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: Preventive effects of selenium. PLoS ONE 2012, 7, e39382. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pu, X.P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, X.; Liu, S.; Deng, Q.; Liu, X.; Peng, S.; Li, H.; Liu, J.; Cao, Y. The role of targeting kinase activity by natural products in cancer chemoprevention and chemotherapy. Oncol. Rep. 2015, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Su, J.; Li, L.; Chen, J.; Hu, S.; Zhang, X.; Chen, T. Mechanistic elucidation of apoptosis and cell cycle arrest induced by 5-hydroxymethylfurfural, the important role of ROS-mediated signaling pathways. Food Res. Int. 2014, 66, 186–196. [Google Scholar] [CrossRef]
- Wu, P.; Liu, S.; Su, J.; Chen, J.; Li, L.; Zhang, R.; Chen, T. Apoptosis triggered by isoquercitrin in bladder cancer cells by activating the AMPK-activated protein kinase pathway. Food Funct. 2017, 8, 3707–3722. [Google Scholar] [CrossRef] [PubMed]
- Peramaiyan, R.; Thamaraiselvan, R.; Natarajan, N.; Rajendran, P.; Yutaka, N.; Ikuo, N. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef]
- Meng, X.; Dong, X.; Wang, W.; Yang, L.; Zhang, X.; Li, Y.; Chen, T.; Ma, H.; Qi, D.; Su, J. Natural borneol enhances paclitaxel-induced apoptosis of ESCC cells by inactivation of the PI3K/AKT. J. Food Sci. 2018, 83, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Su, M.; Qiu, W.; Zhang, M.; Guo, Z.; Su, B.; Liu, J.; Li, X.; Zhou, L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int. J. Mol. Sci. 2013, 14, 21215–21226. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, L.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2010, 30, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, R.; Peng, N.; Moore, R.; Arabshahi, A.; Wyss, J.M.; Barnes, S.; Prasain, J.K. Determination of cranberry phenolic metabolites in rats by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6682–6688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Su, J.; Lin, L.; Hu, S.Q.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-Hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Rooij, M.D.; Jong, L.D. Volume change measurements of rice by environmental scanning electron microscopy and stereoscopy. Scanning 2007, 29, 197. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, E.K. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010, 297, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Su, J.; Li, B.; Zhang, X.; Chen, T. Proteomic analysis of G2/M arrest triggered by natural borneol/curcumin in HepG2 cells, the importance of ROS-p53 pathway. J. Agric. Food Chem. 2015, 63, 6440–6449. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Su, J.; Chen, T. Natural borneol enhances bisdemethoxycurcumin-induced cell cycle arrest in the G2/M phase through up-regulation of intracellular ROS in HepG2 cells. Food Funct. 2014, 6, 740–748. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Group | Final Concentration | Hemolysis Inhibition Rate (%) |
|---|---|---|
| VC (positive control) | 80 μM | 96.84 ± 0.0010 a |
| Kaempferol | 20 μM | 81.00 ± 0.3270 b |
| Kaempferol | 40 μM | 93.63 ± 0.0180 a |
| Kaempferol | 80 μM | 94.80 ± 0.0058 a |
| Damaging group (AAPH) | 200 mM | 42.15 ± 0.0025 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. https://doi.org/10.3390/molecules23102592
Wu P, Meng X, Zheng H, Zeng Q, Chen T, Wang W, Zhang X, Su J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules. 2018; 23(10):2592. https://doi.org/10.3390/molecules23102592
Chicago/Turabian StyleWu, Ping, Xiaofeng Meng, Huade Zheng, Qin Zeng, Tianfeng Chen, Wen Wang, Xia Zhang, and Jianyu Su. 2018. "Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer" Molecules 23, no. 10: 2592. https://doi.org/10.3390/molecules23102592