(−)-Epicatechin Reduces Blood Pressure and Improves Left Ventricular Function and Compliance in Deoxycorticosterone Acetate-Salt Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. Systolic Blood Pressure
2.2. Biometric Measurements and Serum Malondialdehyde
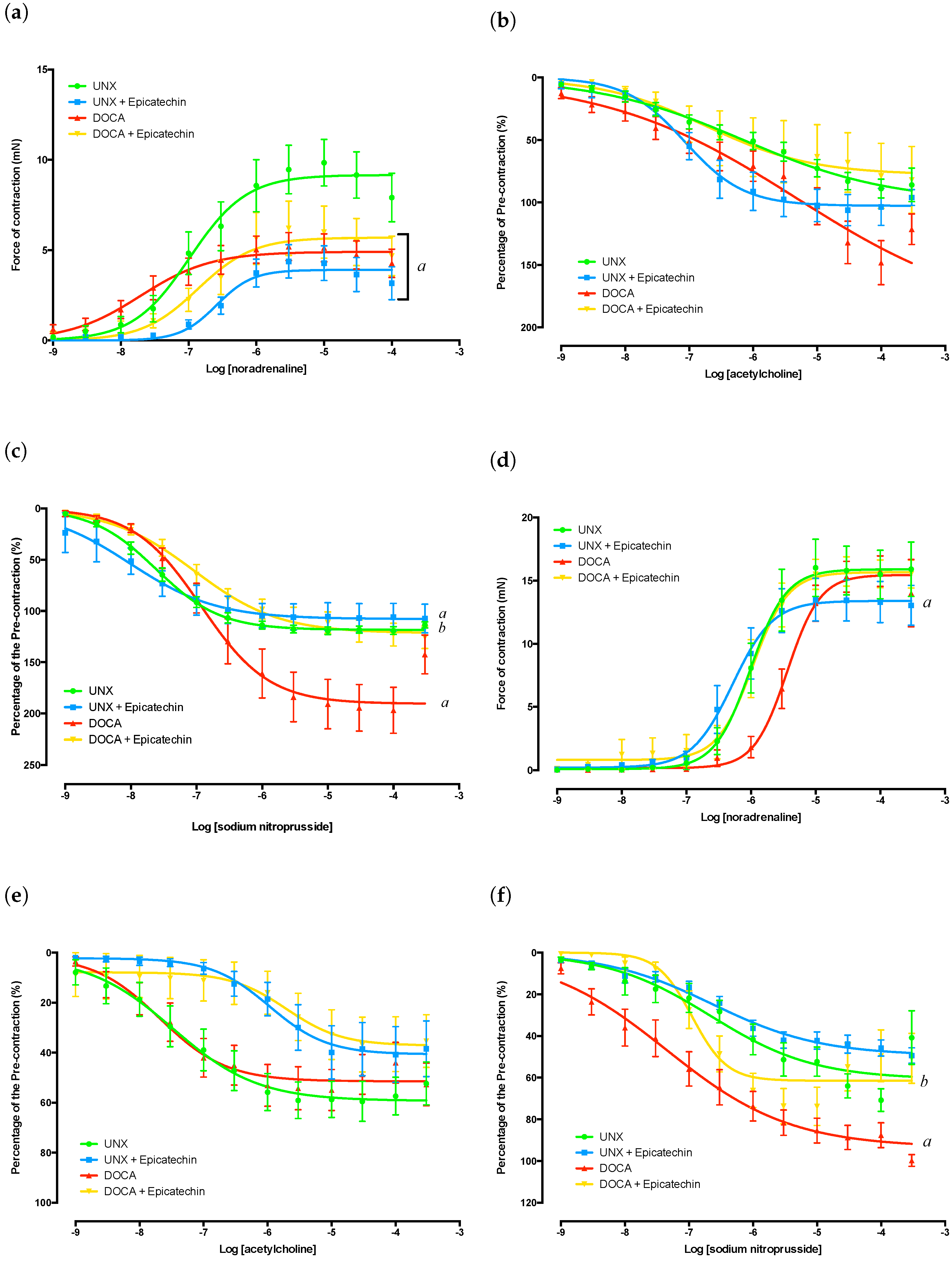
2.3. Thoracic Aorta Functional Analysis
2.4. Mesenteric Artery Functional Analysis
2.5. Cardiac Function
3. Discussion
4. Study Limitations
5. Materials and Methods
5.1. Chemicals and Treatment
5.2. Establishment of Animal Model
5.3. Experimental Design
5.4. Systolic Blood Pressure
5.5. Serum Malondialdehyde Determination
5.6. Thoracic Aorta Organ Baths
5.7. Mesenteric Wire Myograph
5.8. Single-Cell Micro-Electrode
5.9. Langendorff Isolated Heart Preparation
5.10. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| DOCA | Deoxycorticosterone acetate |
| ELISA | Enzyme-link-immunosorbent assay |
| eNOS | Endothelial nitric oxide synthase |
| LV | Left ventricular |
| MDA | Malondialdehyde |
| MMP | Matrix metalloproteinases |
| NA | Noradrenaline |
| NaNO | Sodium nitroprusside |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-B | Nuclear factor kappa B |
| NO | Nitric oxide |
| TLR4 | Toll-like receptor 4 |
| TNF- | Tumour necrosis factor alpha |
| UNX | Uninephrectomy |
References
- Vazquez-Prieto, M.A.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin prevents TNF-α-induced activation of signaling cascades involved and insulin sensitivity in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2012, 527, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.G.; Romero-Perez, D.; Barraza-Hidalgo, M.; Cruz, M.; Rivas, M.; Cortez-Gomez, B.; Ceballos, G.; Villarreal, F. Short- and long-term effects of (−)-epicatechin on myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H761–H767. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.G.; Taub, P.R.; Barraza-Hidalgo, M.; Rivas, M.M.; Zambon, A.C.; Ceballos, G.; Villarreal, F.J. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J. Am. Coll. Cardiol. 2010, 55, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M. (−)-Epicatechin attenuates mitochondrial damage by enhancing mitochondrial multi-marker enzymes, adenosine triphosphate and lowering calcium in isoproterenol induced myocardial infarcted rats. Food Chem. Toxicol. 2013, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. (−)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signalling pathways. Hypertension 2010, 55, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. (−)-Epicatechin induces calcium and translocation independent eNOS activation in arterial endothelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C880–C887. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M. LPS-induced renal inflammation is prevented by (−)-epicatechin in rats. Redox Biol. 2017, 11, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.G.; Andreyev, A.Y.; Ortiz-Vilchis, P.; Petrosyan, S.; Divakaruni, A.S.; Wiley, S.E.; De La Fuente, C.; Perkins, G.; Ceballos, G.; Villarreal, F.; et al. Intravenous (−)-epicatechin reduces myocardial ischemic injury by protecting mitochondrial function. Int. J. Cardiol. 2014, 175, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Sanchez, I.; Aguilar, H.; Ceballos, G.; Villarreal, F. (−)-Epicatechin-induced calcium independent eNOS activation: Roles of HSP90 and AKT. Mol. Cell. Biochem. 2012, 370, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Schewe, T.; Sies, H. (−)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007, 359, 828–833. [Google Scholar] [CrossRef] [PubMed]
- MacRae, K.; Connolly, K.; Vella, R.; Fenning, A. Epicatechin’s cardiovascular protective effects are mediated via opioid receptors and nitric oxide. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- De los Santos, S.; García-Pérez, V.; Hernández-Reséndiz, S.; Palma-Fores, C.; González-Gutiérrez, C.J.; Zazueta, C.; Canto, P.; Coral-Vázquez, R. (−)-Epicatechin induces physiological cardiac growth by activation of the PI3K/Akt pathway in mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, M.; Tsutsumi, Y.M.; Bonds, J.A.; Horikawa, Y.T.; Saldana, M.; Dalton, N.D.; Head, B.P.; Patel, P.M.; Roth, D.M.; Patel, H.H. Dark chocolate receptors: epicatechin-induced cardiac protection is dependent on δ-opioid receptor stimulation. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1604–H1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenning, A.; Harrison, G.; Rose-meyer, R.; Hoey, A.; Brown, L. l-Arginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1408–H1416. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Ooi, S.Y.; Lau, K.; Sernia, C. Cardiac and vascular responses in deoxycorticosterone acetate-salt hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Hoey, A.; Brown, L. Improved cardiovascular function with aminoguanidine in DOCA-salt hypertensive rats. Br. J. Pharmacol. 2006, 148, 902–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, V.; Fenning, A.; Iyer, A.; Hoey, A.; Brown, L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr. Pharm. Biotechnol. 2011, 12, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Wang, H.D.; McNeill, J.R. Role of oxidative stress and nitric oxide in regulation of spontaneous tone in aorta of DOCA-salt hypertensive rats. Br. J. Pharmacol. 2004, 141, 562–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loch, D.; Hoey, A.; Morisseau, C.; Hammock, B.O.; Brown, L. Prevention of hypertension in DOCA-salt rats by an inhibitor of soluble expoxide hydrolase. Cell Biochem. Biophys. 2007, 47, 87–97. [Google Scholar] [CrossRef]
- Mirkovic, S.; Seymour, A.M.L.; Fenning, A.; Strachan, A.; Margolin, S.B.; Taylor, S.M.; Brown, L. Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats. Br. J. Pharmacol. 2002, 135, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Somers, M.J.; Mavromatis, K.; Galis, Z.S.; Harrison, D.G. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 2000, 101, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Chen, C.C.; Chang, N.C. Cardiac sympathetic hyperinnervation in deoxycorticosterone acetate-salt hypertensive rats. Clin. Sci. (Lond.) 2012, 123, 445–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Zarzuelo, M.; Galindo, P.; Quintela, A.; López-Sepúlveda, R.; Romero, M.; Tamargo, J.; Vargas, F.; et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 2012, 52, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Tangsucharit, P.; Takatori, S.; Zamami, Y.; Goda, M.; Pakdeechote, P.; Kawasaki, H.; Takayama, F. Muscarinic acetylcholine receptor M1 and M3 subtypes mediate acetylcholine-induced endothelium-independent vasodilation in rat mesenteric arteries. J. Pharmacol. Sci. 2016, 130, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.; Kerfant, B.G.; Lalevee, N.; Bideaux, P.; Rossier, M.F.; Richard, S.; Gómez, A.M.; Benitah, J.P. Mineralocorticoid receptor antagonism prevents the electrical remodelling that precedes cellular hypertrophy after myocardial infarction. Circulation 2004, 110, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Loch, D.; Hoey, A.; Brown, L. Attenuation of cardiovascular remodelling in DOCA-salt rats by the Vasopeptidase inhibitor, Omapatrillat. Clin. Exp. Hypertens. 2006, 28, 475–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loch, D.; Levick, S.; Hoey, A.; Brown, L. Rosuvastatin attenuates hypertension-induced cardiovascular remodeling without affecting blood pressure in DOCA-salt hypertensive rats. J. Cardiovasc. Pharmacol. 2006, 47, 396–404. [Google Scholar] [PubMed]
- Prince, P.S.M. (−)-Epicatechin prevents alterations in lysosomal glycohydrolases, cathepsins and reduces myocardial infarct size in isoproterenol-induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 706, 63–69. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| UNX | UNX + E | DOCA | DOCA + E | |
|---|---|---|---|---|
| Body mass (BM) (g) | ||||
| 444 ± 13 | 452 ± 14 | 351 ± 10 a | 343 ± 15 a | |
| Organ mass (g/kg BM) | ||||
| Whole heart | 2.64 ± 0.14 | 3.28 ± 0.07 a | 3.42 ± 0.12 a | 4.01 ± 0.13 a,b |
| Left ventricle | 2.15 ± 0.12 | 2.74 ± 0.07 a | 2.91 ± 0.10 a | 3.48 ± 0.12 a,b |
| Left ventricle ratio | 0.81 ± 0.01 | 0.83 ± 0.01 | 0.86 ± 0.01 a | 0.87 ± 0.01 a |
| Right ventricle | 0.49 ± 0.03 | 0.55 ± 0.02 | 0.43 ± 0.05 | 0.53 ± 0.03 |
| Liver | 33.3 ± 0.8 | 32.8 ± 0.6 | 37.4 ± 1.4 a | 38.6 ± 1.4 a |
| Kidney | 4.88 ±0.10 | 4.92 ± 0.05 | 8.31 ± 0.33 a | 7.86 ± 0.19 a |
| Spleen | 266 ± 0.11 | 2.86 ± 0.15 | 3.11 ± 0.16 | 3.16 ± 0.38 |
| Blood pressure (mmHg) | ||||
| 0 Weeks | 119 ± 6 | |||
| 2 Weeks | 116 ± 6 | 132 ± 9 | 162 ± 11 a | 134 ± 28 |
| 4 Weeks | 130 ± 6 | 104 ± 5 a | 194 ± 5 a | 147 ± 6 a,b |
| Biochemistry | ||||
| MDA (pmol/mL) | 125.5 ± 4.1 | 107.3 ± 8.3 | 183.6 ± 22.6 a | 134.6 ± 4.8 b |
| UNX | UNX + E | DOCA | DOCA + E | |
|---|---|---|---|---|
| Aorta | ||||
| Noradrenaline | -6.73 ± 0.20 | -6.46 ± 0.10 | -7.57 ± 0.19 | -6.86 ± 0.17 |
| Acetylcholine | -6.35 ± 0.23 | -6.83 ± 0.19 | -6.26 ± 0.31 | -5.81 ± 0.36 |
| Sodium nitroprusside | -7.59 ± 0.11 | -8.07 ± 0.14 | -6.81 ± 0.12 | -6.92 ± 0.15 |
| Mesenteric | ||||
| Noradrenaline | -5.93 ± 0.14 | -6.24 ± 0.12 | -5.46 ± 0.10 | -6.10 ± 0.25 |
| Acetylcholine | -6.71 ± 0.17 | -6.38 ± 0.52 | -7.05 ± 0.28 | -5.69 ± 0.25 |
| Sodium nitroprusside | -6.27 ± 0.26 | -6.66 ± 0.21 | -7.20 ± 0.23 | -6.28 ± 0.40 |
| Aorta | Mesenteric | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | SS | MS | F | p-Value | DF | SS | MS | F | p-Value | |
| Noradrenaline | ||||||||||
| Epicatechin | 1 | 2.619 | 2.619 | 6.917 | 0.0121 | 1 | 2.534 | 2.534 | 9.575 | 0.003 |
| DOCA-salt | 1 | 4.124 | 4.124 | 10.89 | 0.0020 | 1 | 1.031 | 1.031 | 3.896 | 0.054 |
| Interaction | 1 | 0.530 | 0.530 | 1.400 | 0.2437 | 1 | 0.295 | 0.295 | 1.114 | 0.297 |
| Residual | 40 | 15.15 | 0.379 | 44 | 11.64 | 0.2646 | ||||
| Acetylcholine | ||||||||||
| Epicatechin | 1 | 0.003 | 0.003 | 0.003 | 0.9579 | 1 | 9.461 | 9.461 | 12.11 | 0.001 |
| DOCA-salt | 1 | 3.508 | 3.508 | 3.925 | 0.0541 | 1 | 0.034 | 0.034 | 0.044 | 0.835 |
| Interaction | 1 | 2.377 | 2.377 | 2.660 | 0.1104 | 1 | 1.707 | 1.707 | 2.185 | 0.147 |
| Residual | 42 | 37.53 | 0.894 | 41 | 32.02 | 0.781 | ||||
| Sodium nitroprusside | ||||||||||
| Epicatechin | 1 | 1.052 | 1.052 | 5.114 | 0.0285 | 1 | 0.783 | 0.783 | 0.897 | 0.349 |
| DOCA-salt | 1 | 11.23 | 11.23 | 54.58 | <0.0001 | 1 | 0.830 | 0.830 | 0.952 | 0.335 |
| Interaction | 1 | 0.411 | 0.411 | 1.998 | 0.1643 | 1 | 4.785 | 4.785 | 5.485 | 0.024 |
| Residual | 46 | 12.39 | 0.2058 | 41 | 35.77 | 0.872 | ||||
| UNX | UNX + E | DOCA | DOCA + E | |
|---|---|---|---|---|
| Electrophysiological Measurements | ||||
| APD 20% (ms) | 11.90 ± 0.56 | 13.26 ± 1.15 | 23.77 ± 2.81 a | 22.58 ± 4.21 a |
| APD 50% (ms) | 18.63 ± 1.34 | 22.89 ± 2.79 | 46.93 ± 6.36 a | 45.96 ± 10.40 a |
| APD 90% (ms) | 54.80 ± 5.26 | 85.11 ± 8.82 | 120.3 ± 10.51 a | 122.1 ± 17.14 a |
| RMP (mV) | −63.15 ± 4.61 | −52.89 ± 4.78 | −58.43 ± 3.28 | −53.51 ± 4.38 |
| APA (mV) | 58.07 ± 3.00 | 64.48 ± 5.05 | 60.63 ± 4.78 | 69.16 ± 5.03 |
| Force (mN) | 1.33 ± 0.29 | 1.33 ± 0.41 | 1.88 ± 4.88 | 2.62 ± 0.71 |
| dF/dT (V/s) | 0.38 ± 0.08 | 0.44 ± 0.08 | 0.52 ± 0.16 | 0.75 ± 0.18 |
| dV/dT (V/s) | 14.19 ± 0.56 | 16.01 ± 0.48 | 14.73 ± 1.22 | 15.30 ± 1.18 |
| Langendorff Isolated Heart Measurements | ||||
| Diastolic Pressure (mmHg) | 10.71 ± 0.45 | 10.43 ± 0.52 | 10.78 ± 0.47 | 9.29 ± 0.34 |
| Ventricular volume (L) | 27 ± 4 | 48 ± 11 a | 22 ± 3 a | 44 ± 6 a,b |
| Developed Pressure (mmHg) | 133 ± 17 | 113 ± 13 | 81 ± 16 a | 131 ± 13 b |
| +dP/dT (mmHg/s) | 2312 ± 336 | 2067 ± 244 | 1409 ± 283 a | 2580 ± 430 b |
| −dP/dT (mmHg/s) | −1628 ± 259 | −1500 ± 187 | −1031 ± 226 a | −1827 ± 167 b |
| End Systolic Pressure (mmHg) | 135 ± 18 | 123 ± 13 | 92 ± 16 a | 140 ± 12 b |
| Stiffness (k) | 30.33 ± 1.28 | 30.30 ± 2.07 | 33.93 ± 1.01 a | 27.53 ± 1.54 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, D.; Connolly, K.; Batacan, R.; Ryan, K.; Vella, R.; Fenning, A. (−)-Epicatechin Reduces Blood Pressure and Improves Left Ventricular Function and Compliance in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Molecules 2018, 23, 1511. https://doi.org/10.3390/molecules23071511
Jackson D, Connolly K, Batacan R, Ryan K, Vella R, Fenning A. (−)-Epicatechin Reduces Blood Pressure and Improves Left Ventricular Function and Compliance in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Molecules. 2018; 23(7):1511. https://doi.org/10.3390/molecules23071511
Chicago/Turabian StyleJackson, Douglas, Kylie Connolly, Romeo Batacan, Kimberly Ryan, Rebecca Vella, and Andrew Fenning. 2018. "(−)-Epicatechin Reduces Blood Pressure and Improves Left Ventricular Function and Compliance in Deoxycorticosterone Acetate-Salt Hypertensive Rats" Molecules 23, no. 7: 1511. https://doi.org/10.3390/molecules23071511





